Sugar’s Hidden World: Decoding Its Atomic Structure Now!
Carbohydrates, a fundamental class of biomolecules, exhibit a diverse range of properties directly determined by sugar atomic structure. X-ray crystallography, a powerful analytical technique, allows scientists to visualize and understand the intricate three-dimensional arrangement of atoms within sugar molecules. The International Commission on Nomenclature in Chemistry (IUPAC) provides standardized nomenclature rules critical for accurately describing and communicating information about the composition and configuration of different sugars. The work of scientists like Emil Fischer, a pioneer in carbohydrate chemistry, laid the groundwork for our current understanding of how atomic arrangements influence the chemical behavior of these essential compounds, including the critical understanding of sugar atomic structure.
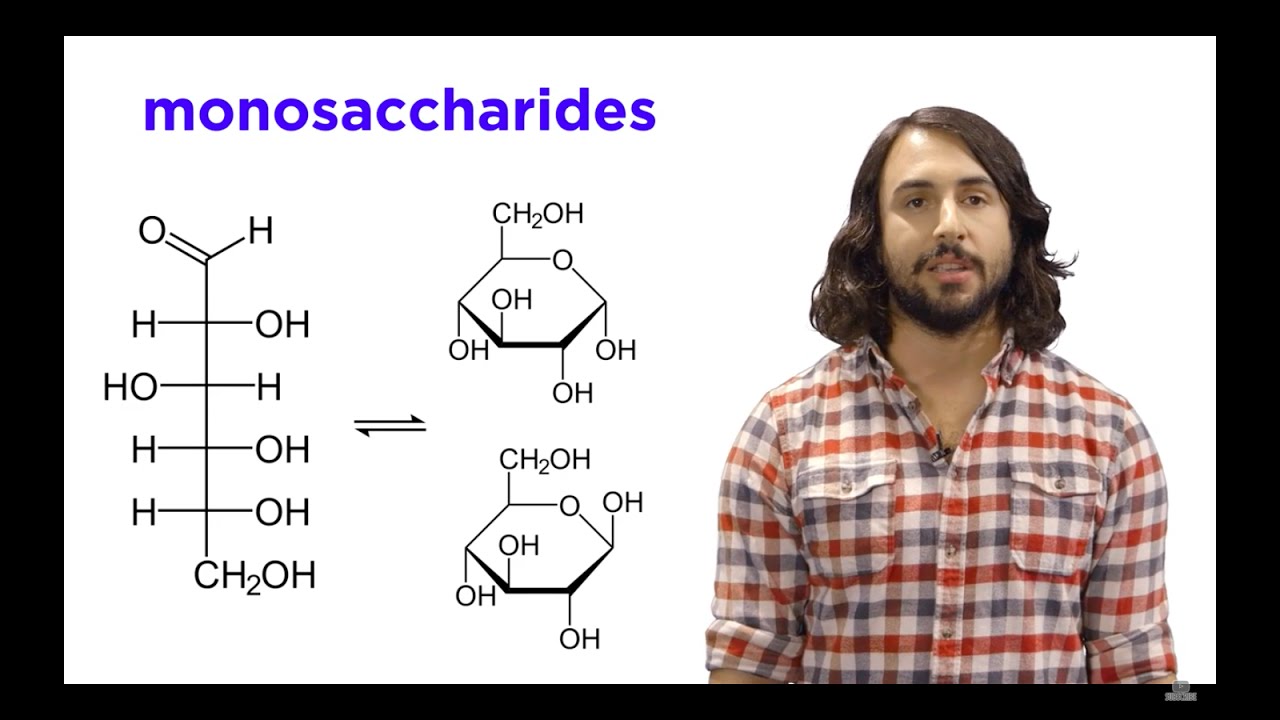
Image taken from the YouTube channel Professor Dave Explains , from the video titled Carbohydrates Part 1: Simple Sugars and Fischer Projections .
Decoding Sugar’s Atomic Structure: An Informative Layout
This outline provides a detailed structure for an article exploring the atomic structure of sugar, with a focus on clarity and accessibility.
Introduction: Setting the Stage for Sugar’s Atomic Secrets
- Engaging Opening: Start with a relatable anecdote or question about sugar’s ubiquity in our lives, briefly mentioning its chemical complexity. For example: "We sprinkle it in our coffee, bake it into our cakes, and find it hidden in countless processed foods. But have you ever stopped to consider what sugar actually is, at its most fundamental level?"
- Defining "Sugar": Clarify what we mean by "sugar" in the context of the article. While many substances are technically sugars, focus on common examples like sucrose (table sugar), glucose, and fructose.
- The Importance of Understanding the Atomic Structure: Highlight why understanding the sugar atomic structure is crucial. Mention aspects like:
- How the structure dictates its properties (sweetness, solubility, etc.)
- How it interacts with our bodies.
- Its role in various chemical reactions.
- Article Overview: Briefly outline the topics to be covered in the following sections.
Unveiling the Building Blocks: Atoms and Chemical Bonds
- The Atom: A Quick Recap: Remind readers about the basic structure of an atom: protons, neutrons, and electrons. Explain how these subatomic particles determine an element’s identity and behavior.
- Protons and Atomic Number: Explain how the number of protons defines the element.
- Electrons and Bonding: Briefly touch upon how electrons are involved in forming chemical bonds.
- Essential Elements in Sugar: Introduce the key elements that make up sugar molecules: Carbon (C), Hydrogen (H), and Oxygen (O).
- Carbon’s Special Role: Explain carbon’s unique ability to form long chains and complex structures, essential for organic molecules like sugars.
- Chemical Bonds: The Glue Holding It All Together: Explain the two main types of chemical bonds relevant to sugar: covalent bonds.
- Covalent Bonds: Describe how atoms share electrons to form stable bonds, and how these bonds are essential for sugar’s structure. Differentiate between single and double bonds, explaining how they affect molecule shape.
Dissecting the Sugar Molecule: Atomic Arrangement
- General Formula of Sugars: Introduce the general formula for carbohydrates (CnH2nOn) and explain what it means.
- Monosaccharides: The Simplest Sugars: Focus on glucose and fructose as primary examples.
- Glucose (C6H12O6):
- Ring Structure: Explain how glucose typically exists in a cyclic (ring) form, showcasing a simplified diagram illustrating the carbon-oxygen ring structure.
- Hydroxyl Groups (-OH): Describe the location and importance of the hydroxyl groups attached to the carbon atoms. These are crucial for solubility and reactivity.
- Alpha and Beta Isomers: Briefly touch upon the concept of alpha and beta glucose, explaining how they differ in the position of the -OH group on carbon 1. A simple diagram can illustrate the difference.
- Fructose (C6H12O6):
- Structural Isomer of Glucose: Explain that fructose has the same chemical formula as glucose but a different arrangement of atoms (structural isomer).
- Ring Structure (Furanose): Show how fructose also forms a ring structure, but a slightly different one (furanose ring).
- Sweetness Perception: Briefly mention how fructose’s unique atomic arrangement contributes to its higher sweetness compared to glucose.
- Glucose (C6H12O6):
- Disaccharides: Two Sugars Joined Together: Focus on sucrose (table sugar) as the primary example.
- Sucrose (C12H22O11):
- Formation: Explain how sucrose is formed from the combination of glucose and fructose through a dehydration reaction (removal of water).
- Glycosidic Bond: Describe the glycosidic bond that links the two monosaccharides. A diagram showing the bond formation would be helpful.
- Diagram of Sucrose Structure: Present a clear diagram illustrating the sucrose molecule, showing the connected glucose and fructose rings.
- Sucrose (C12H22O11):
- Visual Aids: Throughout this section, utilize molecular diagrams or 3D renderings to visually represent the atomic structure of each sugar. Focus on clear labeling of atoms (C, H, O) and bonds.
Factors Affecting Sugar Structure and Properties
- Isomerism: Explain the concept of isomers in more detail.
- Structural Isomers: Reiterate that glucose and fructose are structural isomers.
- Stereoisomers: Briefly introduce the concept of stereoisomers (molecules with the same chemical formula and connectivity but different spatial arrangements), although a detailed explanation may be too complex for the target audience. Focus on the importance of stereochemistry in determining sugar’s interaction with enzymes and taste receptors.
- Hydrogen Bonding: Explain how the hydroxyl groups (-OH) in sugar molecules allow them to form hydrogen bonds with water, contributing to their solubility.
- Temperature and Stability: Discuss how temperature can affect the stability of sugar molecules. For example, caramelization involves the breakdown and rearrangement of sugar molecules at high temperatures.
The Impact of Atomic Structure on Biological Function
- Interaction with Taste Receptors: Explain, at a basic level, how the shape of sugar molecules allows them to bind to taste receptors on our tongues, triggering the sensation of sweetness.
- Energy Source: Describe how the body breaks down sugar molecules to release energy through cellular respiration. Focus on how the sugar atomic structure is crucial for these metabolic processes.
- Building Blocks for Complex Carbohydrates: Explain how simple sugars like glucose can be linked together to form complex carbohydrates (polysaccharides) like starch and cellulose, which serve as energy storage and structural materials in plants.
- Insulin and Glucose Transport: Briefly mention the role of insulin in facilitating the uptake of glucose from the bloodstream into cells. This highlights the interaction of sugar with other biological molecules.
FAQs: Understanding Sugar’s Atomic Structure
Here are some frequently asked questions to help you better grasp the atomic structure of sugar and its significance.
What elements make up the atomic structure of sugar?
The atomic structure of common sugars like sucrose, glucose, and fructose consists primarily of carbon (C), hydrogen (H), and oxygen (O) atoms. These elements are arranged in specific configurations to form the distinct molecules we recognize as sugar.
Why is understanding sugar’s atomic structure important?
Understanding the atomic structure of sugar is crucial for comprehending its properties, such as sweetness, solubility, and how it interacts with the human body. It allows scientists to develop better sweeteners, understand metabolic processes, and even create new materials.
What’s the difference in atomic structure between different types of sugar?
While all sugars contain carbon, hydrogen, and oxygen, the way these atoms are arranged and bonded together dictates the type of sugar. For instance, glucose and fructose have the same chemical formula but different atomic arrangements, leading to varying sweetness levels. Sucrose is formed when a molecule of glucose is chemically bonded to a molecule of fructose.
How does the sugar atomic structure affect how our body processes it?
The specific atomic structure of sugar directly impacts how enzymes in our body break it down. Different sugar atomic structures require different enzymes and metabolic pathways, influencing blood sugar levels and overall energy availability. This knowledge informs dietary recommendations and the development of treatments for metabolic disorders.
So, that’s a glimpse into the fascinating world of sugar atomic structure! Hopefully, you’ve learned something new and can now appreciate the complexity hidden within that spoonful of sugar.