Weak Bases & pH: Unlock the Solubility Secret Now!
Solubility, a fundamental property in chemistry, dictates the extent to which a substance dissolves in a solvent. Weak bases, as characterized by Bronsted-Lowry theory, accept protons but do not fully dissociate in water, leading to a pH-dependent solubility behavior. Understanding the influence of acidity, a concept deeply rooted in acid-base equilibria, is crucial for predicting and controlling the dissolution of these compounds. This article explores the relationship where at low ph weak bases are less soluble?, delving into the underlying principles and providing a comprehensive analysis of this phenomenon.
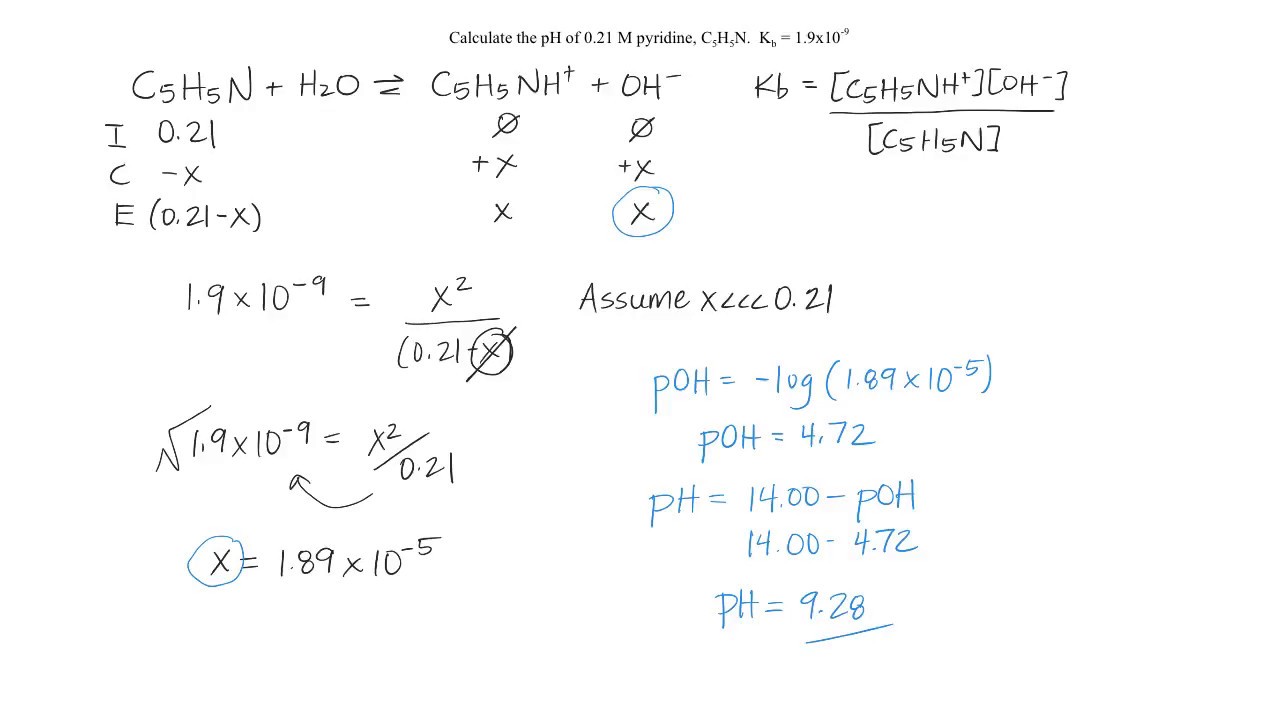
Image taken from the YouTube channel GHC Chemistry , from the video titled pH of a Weak Base .
Unlocking the Solubility Secrets of Weak Bases with pH
The world around us is governed by chemical interactions, many of which hinge on the delicate balance of solubility. Consider, for instance, the targeted delivery of life-saving drugs, where the solubility of a weak base determines its absorption and effectiveness within the body. Or, reflect on the fate of pollutants in our environment, where the solubility of basic compounds dictates their mobility and potential impact on ecosystems. These scenarios, seemingly disparate, are united by a common thread: the profound influence of pH on the solubility of weak bases.
Defining Weak Bases and Their Significance
Weak bases are chemical species that accept protons (H+) from water, resulting in the formation of hydroxide ions (OH-) and a conjugate acid. Unlike strong bases, they do not fully ionize in aqueous solutions, establishing an equilibrium between the unprotonated and protonated forms. This incomplete ionization is a defining characteristic, making their behavior highly sensitive to environmental factors, most notably pH.
The importance of weak bases stems from their prevalence in various fields. Many pharmaceuticals, agrochemicals, and environmental contaminants are weak bases. Understanding their behavior is crucial for designing effective drugs, managing environmental risks, and optimizing industrial processes.
The Interplay of pH and Solubility
pH, a measure of the acidity or alkalinity of a solution, profoundly impacts the solubility of weak bases. The pH scale ranges from 0 to 14, with values below 7 indicating acidic conditions, values above 7 indicating alkaline conditions, and 7 representing a neutral solution.
The solubility of a weak base is intricately linked to the concentration of hydrogen ions (H+) in the solution. In acidic conditions (low pH), the increased concentration of H+ drives the protonation of the weak base, forming its conjugate acid. This protonated form is generally more soluble in water than the unprotonated base.
Thesis: The Decisive Influence of Protonation and Acid-Base Equilibrium
The solubility of weak bases is decisively influenced by pH due to protonation and the principles of acid-base equilibrium. Lowering the pH generally increases the solubility of weak bases by shifting the equilibrium towards the formation of the protonated, more soluble form. This relationship has significant implications across diverse scientific and technological domains, underpinning advancements in drug delivery, environmental remediation, and materials science. Understanding and manipulating this relationship is key to unlocking numerous technological advancements and addressing critical challenges.
The pH of a solution sets the stage, dictating whether a weak base will readily dissolve or remain stubbornly insoluble. But to truly appreciate this dance between pH and solubility, we must first delve into the nature of weak bases themselves, understanding what makes them unique and how they behave in aqueous environments.
Understanding Weak Bases: A Deep Dive
Weak bases occupy a fascinating middle ground in the world of acid-base chemistry. Unlike their strong counterparts, which dissociate completely in water, weak bases engage in a more subtle, equilibrium-driven interaction. This incomplete ionization is the key to their pH-dependent solubility.
What Defines a Weak Base?
At its core, a weak base is a chemical species capable of accepting a proton (H+) from another substance, typically water. This proton acceptance leads to the formation of hydroxide ions (OH-) in the solution, increasing its alkalinity.
The defining characteristic of a weak base is its incomplete ionization in water. When a weak base is added to water, only a fraction of its molecules will accept protons and form hydroxide ions.
The remaining molecules persist in their original, unprotonated form. This creates a dynamic equilibrium between the base, water, its conjugate acid, and hydroxide ions.
Common Examples of Weak Bases
Many everyday compounds fall into the category of weak bases. Ammonia (NH3), a common ingredient in household cleaners and fertilizers, is perhaps the most well-known example.
Amines, organic compounds containing nitrogen atoms, are another large and important class of weak bases. These compounds are prevalent in pharmaceuticals, dyes, and various biological molecules.
Other notable examples include pyridine, aniline, and various alkaloids found in plants. The diverse nature of these compounds highlights the broad relevance of weak base chemistry.
The Dissociation of Weak Bases in Water
To truly understand the behavior of weak bases, we must examine their dissociation process in detail.
The Equilibrium Reaction
When a weak base (represented as B) is added to water, it undergoes a reversible reaction:
B(aq) + H2O(l) ⇌ BH+(aq) + OH-(aq)
This equation illustrates the dynamic equilibrium between the unprotonated base (B), water, the protonated form of the base (BH+), and hydroxide ions (OH-). The double arrow (⇌) signifies that the reaction proceeds in both directions simultaneously.
The position of this equilibrium determines the extent to which the base is protonated and, consequently, the concentration of hydroxide ions in the solution.
The Base Dissociation Constant (Kb)
The extent of dissociation of a weak base is quantified by its base dissociation constant (Kb). This equilibrium constant reflects the relative concentrations of the reactants and products at equilibrium.
For the general reaction above, the Kb is defined as:
Kb = [BH+][OH-] / [B]
A larger Kb value indicates a greater degree of dissociation, meaning the base is "stronger" in the weak base sense. Conversely, a smaller Kb value indicates a weaker base with less dissociation.
Kb values are typically small for weak bases, reflecting their incomplete ionization. They provide a valuable tool for comparing the relative strengths of different weak bases and predicting their behavior in solution.
Factors Affecting the Strength of Weak Bases
Several factors can influence the strength of a weak base, impacting its Kb value and its degree of ionization. These include:
-
Electron-donating groups: Substituents that donate electron density to the base molecule can enhance its ability to accept a proton, increasing its strength.
-
Electron-withdrawing groups: Conversely, substituents that withdraw electron density can decrease the base’s ability to accept a proton, weakening it.
-
Steric effects: Bulky groups near the basic center can hinder protonation, reducing the base’s strength.
-
Resonance: Resonance effects can either increase or decrease the basicity of a molecule, depending on how they affect the availability of electrons for protonation.
Understanding these factors allows us to predict and manipulate the strength of weak bases, which is crucial in various applications, from drug design to chemical synthesis.
The Pivotal Role of pH in Weak Base Solubility
As we begin to explore the nuances of weak base behavior, it’s vital to understand the critical influence of pH. This seemingly simple measure of acidity or alkalinity holds the key to unlocking how and why weak bases dissolve under specific conditions.
The following sections will delve into the fundamental principles of the pH scale, dissect the protonation process that dictates weak base solubility, and illuminate the concept of acid-base equilibrium, thereby building a comprehensive understanding of the intimate relationship between pH and weak base solubility.
Understanding the pH Scale
The pH scale, ranging from 0 to 14, provides a quantitative measure of the acidity or alkalinity of a solution. A pH of 7 indicates neutrality, signifying equal concentrations of hydrogen ions (H+) and hydroxide ions (OH-).
Values below 7 indicate acidity, with lower values representing higher concentrations of H+ ions. Conversely, values above 7 indicate alkalinity or basicity, reflecting higher concentrations of OH- ions.
This logarithmic scale means that each unit change in pH represents a tenfold change in the concentration of H+ or OH- ions. For instance, a solution with a pH of 3 has ten times the concentration of H+ ions compared to a solution with a pH of 4.
How pH Affects Protonation
The protonation of a weak base is a fundamental process that directly impacts its solubility. It is significantly influenced by the pH of the surrounding solution.
The Protonation Process
In acidic solutions (low pH), an abundance of protons (H+) exists. Weak bases, by definition, possess an affinity for protons. When a weak base encounters an acidic environment, it readily accepts a proton, transforming into its conjugate acid.
This protonation process is the cornerstone of the pH-dependent solubility of weak bases. The newly formed conjugate acid often exhibits significantly higher solubility compared to the original, unprotonated weak base.
Protonation Equilibrium
The protonation of a weak base is not an irreversible reaction. Instead, it establishes a dynamic equilibrium between the unprotonated form of the base (B) and its protonated form (BH+).
This equilibrium can be represented as follows:
B + H+ ⇌ BH+
The position of this equilibrium is highly sensitive to the pH of the solution. In highly acidic conditions, the equilibrium shifts towards the right, favoring the formation of the protonated form (BH+).
Conversely, in more alkaline conditions, the equilibrium shifts to the left, favoring the unprotonated form (B). The relative concentrations of B and BH+ at any given pH determine the overall solubility of the weak base.
Solubility and Acid-Base Equilibrium
The concept of acid-base equilibrium is crucial for understanding the behavior of weak bases in solution. This equilibrium governs the dynamic interplay between acids and bases, influencing the concentration of ions present in the solution.
The solubility of a weak base is not a fixed value but rather a variable quantity that depends on the position of its acid-base equilibrium. By manipulating the pH of the solution, we can effectively control this equilibrium and, consequently, the solubility of the weak base.
The relationship between pH, protonation, and solubility is the key to harnessing the unique properties of weak bases in various applications, from drug delivery to environmental remediation.
The Interplay: pH and Solubility of Weak Bases
Having established the fundamental principles of pH, protonation, and acid-base equilibrium, we can now explore their combined effect on the solubility of weak bases. The interplay between these factors dictates whether a weak base will readily dissolve in a solution or remain precipitated. This understanding is crucial in various fields, from pharmaceutical formulation to environmental remediation.
At Low pH, Weak Bases Are More Soluble
The solubility of weak bases exhibits a distinct pH dependence. In acidic environments (low pH), these compounds generally demonstrate enhanced solubility. This phenomenon arises from the increased protonation of the weak base, which shifts the equilibrium towards the formation of its more soluble, charged conjugate acid.
The Common Ion Effect and Protonation
At a low pH, the high concentration of hydrogen ions (H+) drives the protonation of the weak base. The weak base, denoted as "B," accepts a proton (H+) to form its conjugate acid, BH+.
B(aq) + H+(aq) ⇌ BH+(aq)
This protonation reaction is governed by the equilibrium constant. As the concentration of H+ increases (lower pH), the equilibrium shifts to the right, favoring the formation of BH+. This shift is an example of the common ion effect, where the presence of a common ion (H+) influences the equilibrium of the weak base.
Increased Solubility at Lower pH
The formation of the protonated form (BH+) significantly increases the overall solubility of the weak base. While the unprotonated form (B) may have limited solubility in water, the protonated form, being charged, interacts more favorably with water molecules, leading to its increased dissolution.
In essence, by lowering the pH, we are effectively converting the poorly soluble weak base into its more soluble salt form, BH+. This principle is widely exploited in pharmaceutical formulations to enhance the bioavailability of weakly basic drugs.
The Role of Le Chatelier’s Principle
Le Chatelier’s Principle provides a framework for understanding how changes in pH affect the equilibrium and solubility of weak bases. The principle states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress.
In the context of weak base solubility, the "stress" is the addition of H+ ions (lowering the pH). The system relieves this stress by shifting the equilibrium towards the formation of the protonated form (BH+), which consumes the added H+ ions.
This shift, in turn, increases the solubility of the weak base, as more of it is converted into its soluble, protonated form. Conversely, if the pH is raised (decreasing the concentration of H+ ions), the equilibrium will shift to the left, favoring the unprotonated form (B), potentially leading to precipitation if the solution becomes supersaturated with respect to B.
Solubility Product (Ksp) and pH
The solubility product (Ksp) represents the equilibrium constant for the dissolution of a solid compound in water. While Ksp is a constant value for a given compound at a specific temperature, the actual solubility of the compound can vary significantly depending on the pH of the solution.
For a simple salt, the Ksp is defined as the product of the ion concentrations at saturation. However, for weak bases, the solubility is not solely determined by the Ksp. The pH-dependent protonation equilibrium also plays a crucial role.
Even though the Ksp remains constant, the concentration of the unprotonated form (B) at saturation will be lower at low pH values because much of the weak base has been converted to its soluble, protonated form (BH+). Therefore, while Ksp provides a fundamental measure of the compound’s intrinsic solubility, the observed solubility is modulated by the pH of the environment.
The influence of pH on weak base solubility, particularly as governed by protonation, reveals the importance of maintaining a stable pH environment. This is where the concept of buffers becomes crucial, acting as guardians against drastic pH fluctuations and, consequently, ensuring predictable weak base solubility.
Buffers: Stabilizing pH and Influencing Solubility
Buffers are solutions that resist changes in pH upon the addition of small amounts of acid or base. They are essential in various chemical and biological systems where maintaining a stable pH is critical. Buffers exert a powerful influence on the solubility of weak bases. Their capacity to maintain a steady pH directly impacts the equilibrium between the protonated and unprotonated forms of a weak base, affecting its dissolution properties.
Buffers are typically composed of a weak acid and its conjugate base, or a weak base and its conjugate acid. These components work in tandem to neutralize added acids or bases, minimizing pH changes.
For example, a common buffer system is acetic acid (CH3COOH) and its conjugate base, acetate (CH3COO-). When an acid is added to this buffer, the acetate ions react with the excess H+ ions, forming acetic acid and thus neutralizing the impact of the acid.
Conversely, when a base is added, the acetic acid neutralizes the excess OH- ions, forming acetate ions and water. This dynamic equilibrium maintains the pH within a relatively narrow range.
The Effect of Buffers on Solubility
The effectiveness of a buffer in controlling pH has direct implications for the solubility of weak bases.
Maintaining a Stable pH
Weak base solubility is highly sensitive to pH variations, as discussed earlier. Buffers play a critical role in maintaining a stable pH, which in turn, dictates the proportion of a weak base that exists in its soluble, protonated form.
By resisting pH changes, buffers ensure that the equilibrium between the unprotonated weak base (B) and its protonated form (BH+) remains relatively constant.
This stability prevents drastic shifts in solubility, which can be vital in applications where consistent dissolution is required.
Applications of Buffers in Solubility Control
The ability of buffers to control the solubility of weak bases is particularly important in pharmaceutical formulations.
Pharmaceutical Formulations
Many drugs are weak bases, and their effectiveness depends on their ability to dissolve properly in the body. Pharmaceutical scientists often use buffers to ensure that these drugs remain soluble throughout their shelf life and are effectively absorbed upon administration.
For instance, a drug formulated as a weak base may be combined with a buffer system that maintains a slightly acidic pH. This promotes protonation of the drug, increasing its solubility in the aqueous environment of the digestive tract.
The choice of buffer and its concentration are carefully optimized to achieve the desired solubility profile while also considering the drug’s stability and potential interactions with other excipients.
The Role of Acids and Hydroxide Ions (OH-)
Acids and hydroxide ions (OH-) play crucial roles in influencing the solubility of weak bases within buffered systems. Buffers, by definition, contain components that can neutralize both acids and bases, helping maintain a stable pH.
When an acid is introduced into the buffered solution, the conjugate base component of the buffer reacts with the excess hydrogen ions (H+), mitigating the pH change. This reaction shifts the equilibrium towards the protonated form of the weak base, thereby increasing its solubility.
Conversely, when hydroxide ions (OH-) are added, the weak acid component of the buffer neutralizes them, preventing the pH from rising excessively. In this scenario, the equilibrium shifts towards the unprotonated form of the weak base, potentially reducing its solubility if the pH increases significantly.
The interplay between the buffer components and the added acids or bases ultimately determines the extent to which the weak base remains soluble. By carefully selecting the buffer system and its concentration, one can effectively control the solubility of weak bases under varying conditions, ensuring optimal performance in pharmaceutical and other applications.
Real-World Applications: Solubility in Action
Understanding the intricate relationship between pH and the solubility of weak bases transcends theoretical knowledge; it is fundamental to a wide array of practical applications. From designing targeted drug delivery systems to remediating contaminated environments, the principles governing this interaction are actively leveraged to achieve specific outcomes.
Pharmaceutical Applications
The pharmaceutical industry heavily relies on manipulating the solubility of weak base drugs to optimize their efficacy and stability. The bioavailability, absorption, and overall therapeutic effect of these drugs are directly influenced by their solubility profiles.
Drug Delivery
Many drugs are weak bases, and their solubility is highly pH-dependent. This characteristic is strategically exploited in drug delivery systems.
For instance, oral medications containing weak bases are often formulated to dissolve more readily in the acidic environment of the stomach. This promotes rapid absorption into the bloodstream.
Conversely, coatings can be applied to these medications to prevent premature dissolution in the stomach. They enable targeted release in the more alkaline environment of the small intestine.
This targeted approach maximizes drug absorption at the desired location while minimizing potential side effects. It also protects the drug from degradation in the stomach.
Formulation Stability
The stability of drug formulations containing weak bases is also significantly influenced by pH. Uncontrolled pH fluctuations can lead to precipitation of the drug, reducing its effectiveness and shelf life.
Pharmaceutical scientists carefully select buffer systems to maintain the pH of the formulation within an optimal range. This ensures that the weak base remains soluble and chemically stable throughout its storage period.
Moreover, the pH can impact the rate of degradation reactions. Maintaining an appropriate pH can slow down these reactions, extending the shelf life of the drug product.
Environmental Applications
The pH-solubility relationship of weak bases also plays a crucial role in environmental science and engineering. Understanding this relationship is critical for addressing issues related to soil contamination and water treatment.
Soil Chemistry
The solubility of basic compounds in soil is highly dependent on pH. Soil pH affects the mobility and bioavailability of various nutrients and contaminants.
In acidic soils, basic compounds tend to be more soluble. This can increase their mobility and potentially lead to groundwater contamination.
Conversely, in alkaline soils, these compounds may precipitate out of solution. This reduces their bioavailability to plants and other organisms.
Understanding these dynamics is essential for managing soil fertility, predicting the fate of pollutants, and developing effective remediation strategies.
Water Treatment
pH adjustment is a common technique used in water treatment processes to remove or neutralize basic contaminants.
By raising the pH of water, basic compounds can be induced to precipitate out of solution. This allows them to be physically removed through filtration or sedimentation.
For example, heavy metals that form basic hydroxides can be effectively removed from wastewater by increasing the pH to a level where these hydroxides become insoluble.
Conversely, adjusting the pH can also be used to dissolve basic compounds that are causing scaling or corrosion problems in water distribution systems.
Careful control of pH is, therefore, crucial for ensuring the safety and quality of drinking water. This also ensures the efficient operation of industrial water systems.
Weak Bases & pH: FAQs
Here are some frequently asked questions about weak bases, pH, and their impact on solubility.
What exactly is a "weak base"?
A weak base is a base that doesn’t completely dissociate into ions when dissolved in water. This means it only partially accepts protons (H+) from water, resulting in a lower concentration of hydroxide ions (OH-) compared to a strong base.
How does pH affect the solubility of weak bases?
The pH of a solution significantly influences the solubility of weak bases. At low pH, weak bases are less soluble because the increased concentration of H+ ions neutralizes the base, shifting the equilibrium towards the protonated form. This protonated form is often more soluble.
Why is understanding the solubility of weak bases important?
Understanding the relationship between pH and weak base solubility is crucial in many fields. This includes drug development (where drug solubility affects bioavailability), environmental science (for contaminant behavior), and chemical synthesis (for controlling reaction conditions).
What’s the difference between solubility and dissociation?
Solubility refers to the amount of a substance that can dissolve in a solvent. Dissociation refers to the extent to which a compound breaks apart into ions when dissolved. While related, a weak base can be soluble to some degree, but still not fully dissociate into its ions. That is, at low ph weak bases are less soluble.
So there you have it! Hopefully, this has shed some light on why at low ph weak bases are less soluble? Keep these concepts in mind, and you’ll be well on your way to mastering the intricacies of chemistry. Happy experimenting!