Tertiary Carbons: A Deep Dive You Can’t Afford To Miss!
The stability of organic molecules is profoundly influenced by the architecture of their carbon framework, and a cornerstone of this understanding lies in the characteristics of tertiary carbons. These carbons, bonded to one hydrogen atom and three other carbon atoms, exhibit unique reactivity patterns crucial in various chemical processes. Understanding Markownikoff’s rule, a fundamental principle in organic chemistry, requires a thorough grasp of tertiary carbons and their behavior during electrophilic addition reactions. The ACS (American Chemical Society) has published extensive research detailing the role of tertiary carbons in complex organic reactions, and such work forms the basis for further analysis in industrial applications, especially in relation to petrochemical engineering.
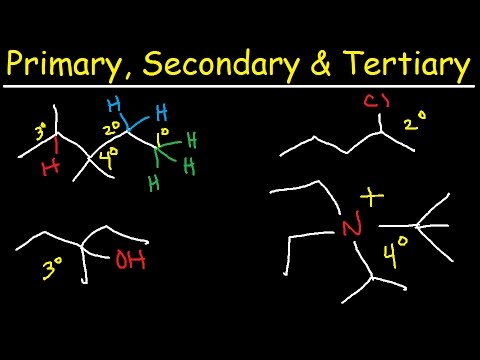
Image taken from the YouTube channel The Organic Chemistry Tutor , from the video titled Primary, Secondary, Tertiary, & Quarternary Hydrogen and Carbon Atoms .
Unpacking Tertiary Carbons: Structure for Maximum Understanding
This article layout is designed to provide a comprehensive and easily digestible explanation of tertiary carbons. By focusing on clarity and logical progression, readers will gain a solid understanding of this fundamental organic chemistry concept.
Defining and Identifying Tertiary Carbons
This section should establish the core definition and explain how to locate tertiary carbons within a molecular structure.
Definition of a Tertiary Carbon
Begin with a clear, concise definition: A tertiary carbon is a carbon atom that is bonded to three other carbon atoms. This is crucial for understanding the rest of the information.
Visual Identification
- Use clear diagrams or images to visually demonstrate what a tertiary carbon looks like within different molecular structures. Highlight the tertiary carbon with a distinct color or marker.
- Provide examples of simple alkanes and more complex cyclic structures.
- Illustrate examples where a carbon appears to be tertiary but isn’t, due to heteroatom attachments (e.g., if bonded to three carbons and an oxygen).
Systematic Nomenclature
Explain how the location of a tertiary carbon can be incorporated into the IUPAC naming system, even though it isn’t directly named in most situations. Instead, explain how the position affects the naming of substituents or functional groups.
Properties and Reactivity of Tertiary Carbons
This section will delve into the unique properties and reactivity patterns associated with tertiary carbons.
Steric Hindrance
- Explain how the presence of three bulky carbon substituents around a tertiary carbon creates steric hindrance.
- Describe the impact of steric hindrance on the accessibility of the carbon atom for chemical reactions.
- Use visual aids showing how the bulky groups impede approaching reagents.
Stability of Carbocations
- Explain the concept of carbocations and how they are formed.
- Detail why tertiary carbocations are more stable than secondary or primary carbocations.
- Explain the role of hyperconjugation in stabilizing tertiary carbocations. Use diagrams to illustrate hyperconjugation.
Reactivity in SN1 Reactions
- Explain the SN1 reaction mechanism.
- Illustrate how the stability of the tertiary carbocation intermediate favors SN1 reactions over SN2 reactions at tertiary carbons.
- Show the reaction mechanism of a typical SN1 reaction involving a tertiary alkyl halide.
Reactivity in E1 Reactions
- Explain the E1 reaction mechanism.
- Discuss how the formation of a stable tertiary carbocation facilitates E1 elimination reactions.
- Show the reaction mechanism of a typical E1 reaction.
Examples and Applications
This section offers practical examples of compounds containing tertiary carbons and illustrates their importance in various applications.
Common Examples of Molecules with Tertiary Carbons
| Molecule | Description | Significance |
|---|---|---|
| 2-Methylbutane | A simple branched alkane with one tertiary carbon. | Model compound for understanding basic alkane structure. |
| Adamantane | A cage-like molecule with several tertiary carbons in a highly symmetrical arrangement. | Building block in polymer chemistry and pharmaceutical design. |
| Steroids | Complex cyclic molecules with numerous tertiary carbons, defining their overall shape and chemical properties. | Found in hormones, drugs, and other biologically active compounds. |
| Tertiary Butanol | An alcohol with a tertiary carbon directly bonded to a hydroxyl group. | Used as a solvent, intermediate in chemical synthesis, and cleaning agent. |
Applications in Pharmaceuticals
- Describe how tertiary carbons are frequently found in drug molecules.
- Explain how their presence can influence the drug’s binding affinity, metabolic stability, and overall efficacy.
- Provide specific examples of drugs containing tertiary carbons and how their structure affects their biological activity.
Applications in Polymer Chemistry
- Illustrate how tertiary carbons are incorporated into polymer backbones or side chains.
- Explain how the presence of tertiary carbons can affect the polymer’s properties, such as its glass transition temperature, flexibility, and chemical resistance.
- Provide specific examples of polymers containing tertiary carbons.
Frequently Asked Questions About Tertiary Carbons
Here are some common questions about tertiary carbons and their significance in organic chemistry.
What exactly is a tertiary carbon?
A tertiary carbon atom is a carbon atom that is bonded to three other carbon atoms. It is different from primary, secondary, and quaternary carbons, which are bonded to one, two, and four other carbons, respectively. Understanding tertiary carbons is key to predicting a molecule’s reactivity.
Why are tertiary carbons considered more reactive?
Tertiary carbons are more reactive than primary or secondary carbons because the tertiary carbocation formed after a reaction is more stable. This stability arises from hyperconjugation, where electrons from neighboring C-H bonds stabilize the positive charge on the tertiary carbon. The higher stability lowers the activation energy for reactions involving tertiary carbons.
How can I identify tertiary carbons in a molecule?
Look for carbon atoms bonded to three other carbon atoms. It’s crucial to visualize the full structure of the molecule, not just the condensed formula. Be sure to clearly identify all the carbons directly attached to your carbon atom of interest. Tertiary carbons will always have only one hydrogen atom bonded to them (unless they have a positive or negative charge).
What are some real-world applications of understanding tertiary carbons?
The understanding of tertiary carbons is crucial in various fields, including drug design and materials science. For example, predicting the site of reaction in a complex molecule with tertiary carbons can help optimize drug synthesis. Similarly, understanding the reactivity of tertiary carbons can enable the design of more stable and durable polymers.
So, that’s the lowdown on tertiary carbons! Hopefully, this deep dive gave you a clearer picture of how these little guys work. Now you’re equipped to tackle some complex organic chemistry. Good luck, and keep exploring the amazing world of tertiary carbons!