Sulfite Lewis Structure: Unveiling its Secrets!
The sulfite ion (SO3^2-), a fundamental component in various chemical reactions, exhibits a sulfite lewis structure that reveals its bonding arrangement and electronic distribution. Understanding the VSEPR theory is crucial for accurately depicting this structure, as it dictates the molecular geometry. Resonance structures, furthermore, play a significant role in representing the delocalization of electrons within the sulfite lewis structure. The accurate representation of the sulfite lewis structure aids chemists in predicting reactivity and understanding its role in processes like sulfur dioxide scrubbing.
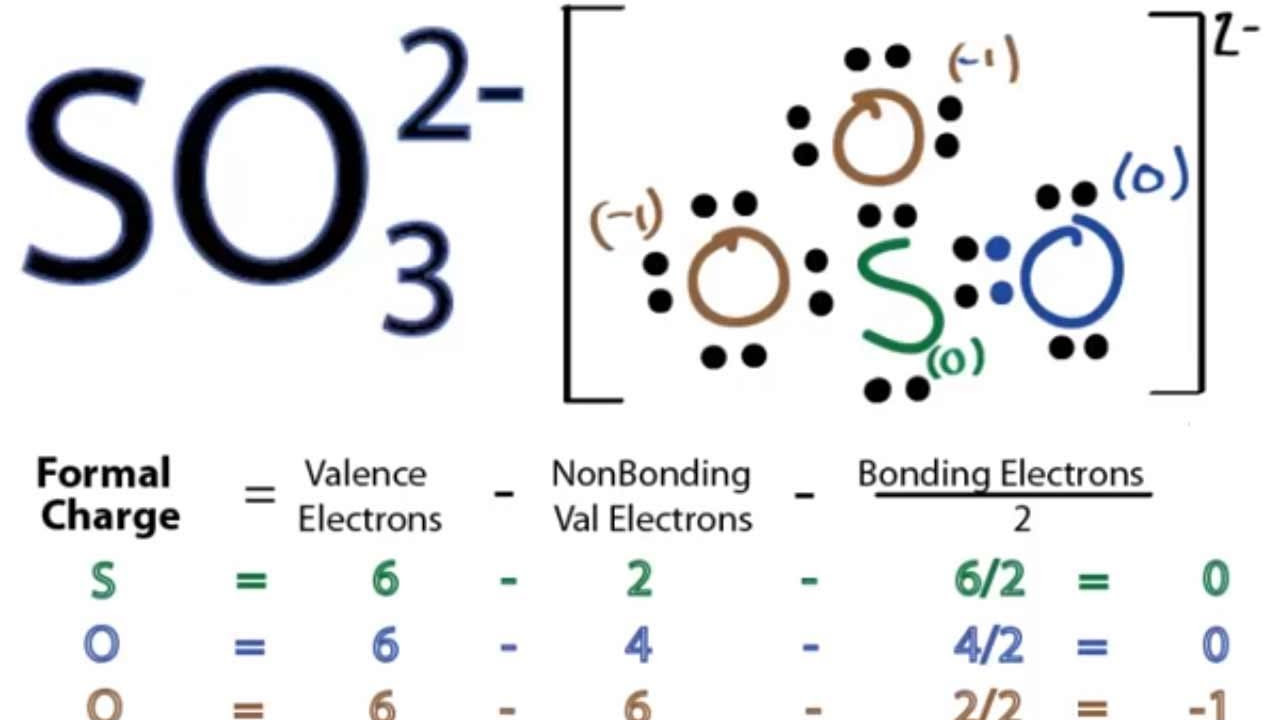
Image taken from the YouTube channel Wayne Breslyn (Dr. B.) , from the video titled SO3 2- Lewis Structure – How to Draw the Lewis Structure for SO3 2- (Sulfite Ion) .
Unveiling the Secrets of the Sulfite Lewis Structure
Understanding the sulfite Lewis structure is crucial for grasping the chemical properties and reactivity of the sulfite ion (SO32-). This explanation will guide you through the process of drawing and interpreting the sulfite Lewis structure, highlighting key aspects like valence electrons, formal charges, and resonance.
Determining Valence Electrons
The first step in constructing a Lewis structure is determining the total number of valence electrons. This is done by summing the valence electrons of each atom in the ion and adding the charge.
- Sulfur (S): Sulfur is in group 16 (or 6A) of the periodic table, so it has 6 valence electrons.
- Oxygen (O): Oxygen is also in group 16 (or 6A), thus each oxygen atom has 6 valence electrons. Since there are three oxygen atoms, the total is 3 * 6 = 18 valence electrons.
- Charge: The sulfite ion has a -2 charge, meaning there are two extra electrons.
Therefore, the total number of valence electrons in the sulfite ion is 6 + 18 + 2 = 26.
Constructing the Skeletal Structure
Next, we need to arrange the atoms in a reasonable skeletal structure. Sulfur is less electronegative than oxygen, so it will be the central atom. The three oxygen atoms will be arranged around the sulfur atom.
The initial skeleton will look like this: O-S-O-O.
Distributing Electrons and Checking Octets
Now, we distribute the 26 valence electrons around the atoms to satisfy the octet rule (or duet rule for hydrogen, which is not present here). We start by placing single bonds between the sulfur and each oxygen atom, using 2 electrons per bond (total of 6 electrons). This leaves us with 26 – 6 = 20 electrons.
Next, we distribute these remaining electrons as lone pairs around the oxygen atoms to satisfy their octets. Each oxygen needs 6 more electrons to complete its octet (3 lone pairs). This requires 3 oxygen atoms * 6 electrons/oxygen = 18 electrons. We now have 20 – 18 = 2 electrons remaining.
These remaining 2 electrons are placed on the central sulfur atom as a lone pair. At this point, all 26 electrons have been used.
Evaluating Formal Charges
To further refine the structure, we calculate the formal charge on each atom. The formal charge is calculated using the following formula:
Formal Charge = (Valence Electrons) – (Non-bonding Electrons) – (1/2 * Bonding Electrons)
- Sulfur: 6 (valence) – 2 (non-bonding) – (1/2 * 6) (bonding) = +1
- Oxygen (singly bonded): 6 (valence) – 6 (non-bonding) – (1/2 * 2) (bonding) = -1
The sum of the formal charges (+1 -1 -1 -1 = -2) equals the overall charge of the sulfite ion.
Resonance Structures and Double Bond Formation
To minimize formal charges, we can form a double bond between one of the oxygen atoms and the sulfur atom. This involves moving one lone pair from an oxygen atom to form a double bond with sulfur. This reduces the formal charge on both the sulfur and that particular oxygen atom to 0.
The formula charge changes with the introduction of the double bond. The oxygen atom forming the double bond now has a formal charge of 0 and the Sulfur also has a formal charge of 0. This creates a better molecular structure than that with all the oxygen atoms with single bonds.
However, since any of the three oxygen atoms could form the double bond, the sulfite ion exhibits resonance. This means that the actual structure is a hybrid of all possible resonance structures. We represent this by drawing all possible structures with single-headed arrows between them.
Consider the oxygen atom forming a double bond with the Sulfur atom, then:
- Sulfur: 6 (valence) – 2 (non-bonding) – (1/2 * 8) (bonding) = 0
- Oxygen (doubly bonded): 6 (valence) – 4 (non-bonding) – (1/2 * 4) (bonding) = 0
- Oxygen (singly bonded): 6 (valence) – 6 (non-bonding) – (1/2 * 2) (bonding) = -1
While all three resonance structures contribute to the overall structure, the true structure is a resonance hybrid.
Sulfite Lewis Structure: Frequently Asked Questions
[This section provides answers to common questions about the sulfite Lewis structure and its properties.]
Why does sulfite (SO3^2-) require resonance structures?
Sulfite (SO3^2-) requires resonance structures because the double bond can be placed on any of the three oxygen atoms. This delocalization of electrons contributes to the stability of the ion. Each resonance structure is a valid representation of the sulfite lewis structure, and the actual structure is a hybrid of all of them.
How do you determine the formal charge on each atom in the sulfite lewis structure?
To determine formal charge, you subtract the number of bonds and non-bonding electrons from the number of valence electrons for each atom. Oxygen with a single bond and three lone pairs has a formal charge of -1. Sulfur, with one double bond, one single bond, and one lone pair, has a formal charge of 0. The sum of the formal charges matches the ion’s overall charge (-2).
What is the VSEPR shape of the sulfite ion, and why?
The sulfite ion (SO3^2-) has a tetrahedral electron domain geometry and a trigonal pyramidal molecular geometry according to VSEPR theory. The sulfur atom has three bonding pairs and one lone pair, which repels the bonding pairs, resulting in a trigonal pyramidal shape instead of a trigonal planar shape. This is crucial for understanding the sulfite lewis structure and its reactivity.
How does the sulfite lewis structure differ from the sulfate lewis structure?
While both are sulfur-containing oxoanions, the key difference lies in the number of oxygen atoms and the overall charge. Sulfite (SO3^2-) has three oxygen atoms and a -2 charge, whereas sulfate (SO4^2-) has four oxygen atoms and a -2 charge. This results in different bonding arrangements and therefore, different Lewis structures and properties. The sulfur atom in sulfate also tends to form more double bonds than in sulfite.
So, there you have it! Hopefully, understanding the sulfite lewis structure doesn’t feel like such a mystery anymore. Now you can confidently tackle those chemistry challenges. Happy studying!