Unlock Chemistry: Standard Element Names Decoded!
The world of chemistry hinges on precise communication, where ambiguity can lead to critical errors. IUPAC (International Union of Pure and Applied Chemistry) acts as the governing body for standardizing chemical nomenclature. This standardization is crucial, especially when researchers at institutions like MIT are collaborating on projects. This article focuses on the standard scientific name by which each element or compound is known, ensuring everyone, even those using tools like ChemDraw, understands precisely what substance is being discussed. Gaining a clear understanding of the standard scientific name by which each element or compound is known is essential for anyone working with chemicals.
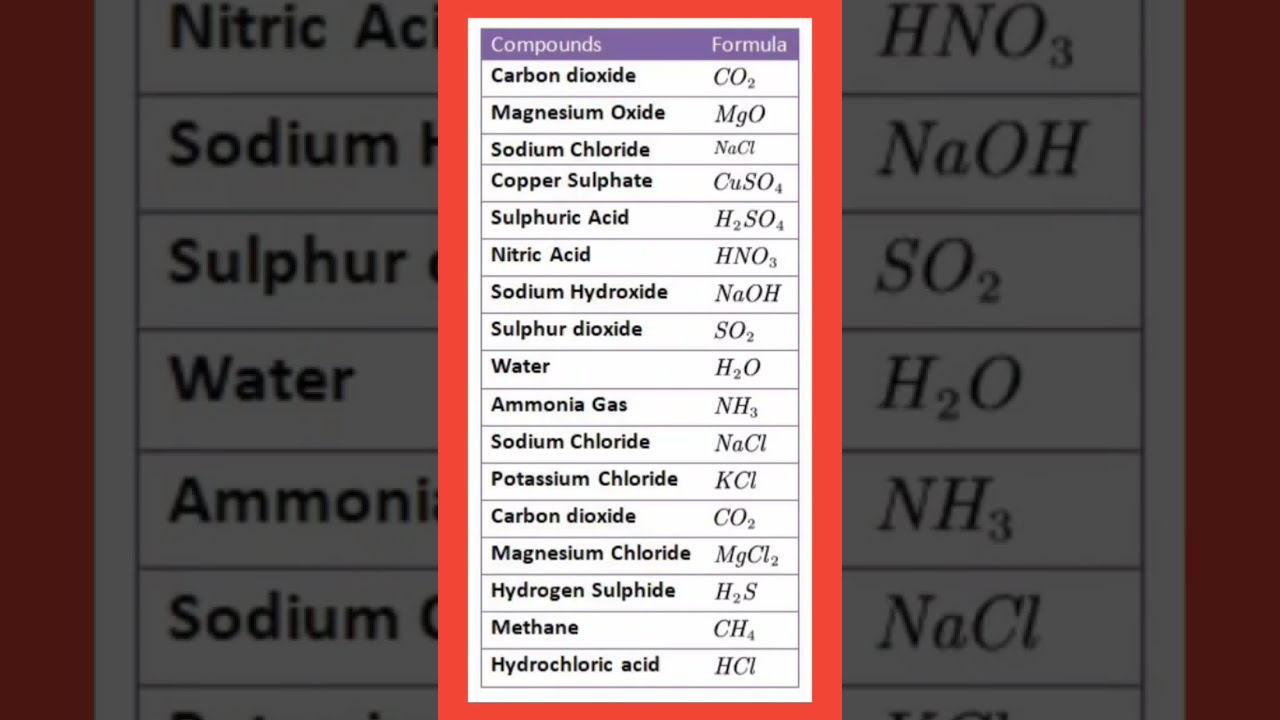
Image taken from the YouTube channel khan ‘ma’am study guide….. , from the video titled #chemistry #compound #formula # .
Unlock Chemistry: Standard Element Names Decoded!
This article aims to explain the "standard scientific name by which each element or compound is known", providing a clear understanding of how these names originate and their importance in chemistry.
The Importance of Standard Scientific Names
Using the standard scientific name is crucial for clear and unambiguous communication in the scientific community. Imagine trying to discuss a chemical reaction if everyone used different names for the reactants!
- Universality: Standard names are internationally recognized, ensuring that scientists worldwide understand each other.
- Precision: They avoid the confusion caused by common names, which can be vague or refer to multiple substances. For instance, "washing soda" refers to a hydrate of sodium carbonate, but doesn’t specify the hydration number.
- Accurate Identification: They provide a consistent and reliable way to identify and refer to elements and compounds.
Understanding Element Names
Origins of Element Names
Element names come from various sources, reflecting historical discoveries, properties, or places.
- Mythology: Many elements, especially in the early days of chemistry, were named after mythological figures.
- Example: Titanium is named after the Titans of Greek mythology.
- Places: Some elements are named after locations, often where they were discovered or are particularly abundant.
- Example: Germanium is named after Germany.
- Properties: Some element names reflect a key property of the element.
- Example: Argon, derived from the Greek word "argos" meaning inactive, refers to its inert nature.
- Scientists: Elements are sometimes named in honor of prominent scientists.
- Example: Einsteinium is named after Albert Einstein.
Element Symbols
Each element has a unique one- or two-letter symbol. These symbols are used in chemical formulas and equations.
- Based on the Name: Most symbols are derived directly from the element’s name.
- Example: Hydrogen’s symbol is H.
- Latin Origins: Some symbols are based on the element’s Latin name.
- Example: Sodium’s symbol is Na (from the Latin "natrium").
- Example: Copper’s symbol is Cu (from the Latin "cuprum").
Understanding Compound Names
Compounds are substances composed of two or more elements chemically bonded together. Naming compounds follows specific rules established by the International Union of Pure and Applied Chemistry (IUPAC). This results in each compound having a "standard scientific name by which it is known".
Types of Compounds and Naming Conventions
-
Ionic Compounds: Compounds formed by the electrostatic attraction between ions (charged atoms or molecules).
- Binary Ionic Compounds (Metal + Nonmetal): Name the metal first, followed by the nonmetal with an "-ide" suffix.
- Example: NaCl is Sodium Chloride.
- Transition Metals: Transition metals can have multiple oxidation states (charges). A Roman numeral in parentheses indicates the charge of the metal ion.
- Example: FeCl2 is Iron(II) Chloride.
- Example: FeCl3 is Iron(III) Chloride.
- Polyatomic Ions: Groups of atoms with an overall charge. Their names are generally memorized.
- Example: Na2SO4 is Sodium Sulfate (SO42- is the sulfate ion).
- Binary Ionic Compounds (Metal + Nonmetal): Name the metal first, followed by the nonmetal with an "-ide" suffix.
-
Molecular Compounds (Nonmetal + Nonmetal): Compounds formed by sharing electrons (covalent bonds).
- Prefixes: Prefixes indicate the number of each type of atom. Common prefixes include:
- Mono- (1), Di- (2), Tri- (3), Tetra- (4), Penta- (5), Hexa- (6), Hepta- (7), Octa- (8), Nona- (9), Deca- (10)
- Example: N2O4 is Dinitrogen Tetroxide. Note that "mono-" is usually omitted for the first element.
- Example: CO is Carbon Monoxide.
- Ending: The second element also ends in "-ide".
- Prefixes: Prefixes indicate the number of each type of atom. Common prefixes include:
-
Acids: Compounds that release hydrogen ions (H+) when dissolved in water.
- Binary Acids (H + Nonmetal): Named with the prefix "hydro-" and the suffix "-ic".
- Example: HCl (aq) is Hydrochloric Acid.
-
Oxyacids (H + Polyatomic Ion containing Oxygen):
- If the polyatomic ion ends in "-ate", the acid name ends in "-ic".
- Example: H2SO4 is Sulfuric Acid (from Sulfate).
- If the polyatomic ion ends in "-ite", the acid name ends in "-ous".
- Example: H2SO3 is Sulfurous Acid (from Sulfite).
- If the polyatomic ion ends in "-ate", the acid name ends in "-ic".
- Binary Acids (H + Nonmetal): Named with the prefix "hydro-" and the suffix "-ic".
Resources for Naming
- IUPAC Nomenclature of Organic Chemistry: IUPAC provides comprehensive rules for naming organic compounds.
- Chemical Handbooks: These often contain tables of common compounds and their names.
- Online Databases: Sites like PubChem provide information on chemical compounds, including their standard names.
FAQs: Understanding Element Names
Here are some frequently asked questions to help you better understand the origins and conventions behind element names.
Why are some element symbols so different from their names?
Many element symbols, like Fe for iron (ferrum), Cu for copper (cuprum), and Au for gold (aurum), come from Latin. This is because Latin was the international language of science when many elements were discovered and named. These symbols represent the standard scientific name by which each element is known in the scientific community, regardless of the common English name.
Who decides on the names of new elements?
The International Union of Pure and Applied Chemistry (IUPAC) is responsible for standardizing chemical nomenclature, terminology, and measurements. When a new element is discovered and its discovery confirmed, the discoverers propose a name and symbol. IUPAC then reviews and officially approves the suggested name, ensuring consistency across the scientific world. The approved name becomes the standard scientific name by which each element or compound is known.
Are there naming conventions for elements?
Yes, there are conventions. Elements can be named after: mythical concepts (like Vanadium, after the Norse goddess Vanadis), places (like Polonium, after Poland), scientists (like Einsteinium, after Albert Einstein), or a property of the element itself (like Argon, from the Greek "argos" meaning inactive). These conventions help categorize elements and reflect their history.
Why is knowing the standard element names important?
Knowing the standard scientific name by which each element or compound is known is crucial for clear communication in science. Using the correct symbol avoids ambiguity, especially since different languages may use different common names for the same element. This standardization ensures that scientists globally understand exactly which element is being discussed in research, publications, and discussions.
Hope this helped demystify the whole *standard scientific name by which each element or compound is known* thing! Now you’re ready to conquer the chemistry world, one element at a time. Happy experimenting!