Plasma H2CO3 Drop: Unveiling Key Causes & Solutions!
The phenomenon of a primary decrease in plasma [h2co3] is a complex interplay of physiological processes, impacting various metabolic pathways. Kidney function, a critical component of acid-base balance, significantly influences bicarbonate regulation in the bloodstream. Furthermore, impairments to respiratory compensation mechanisms can exacerbate reductions in plasma [h2co3] levels. Research conducted by organizations like the Mayo Clinic continues to shed light on the intricacies of this condition. Finally, diagnostic tools such as the arterial blood gas (ABG) test are indispensable for accurately measuring plasma [h2co3] and identifying the underlying causes of its decline.
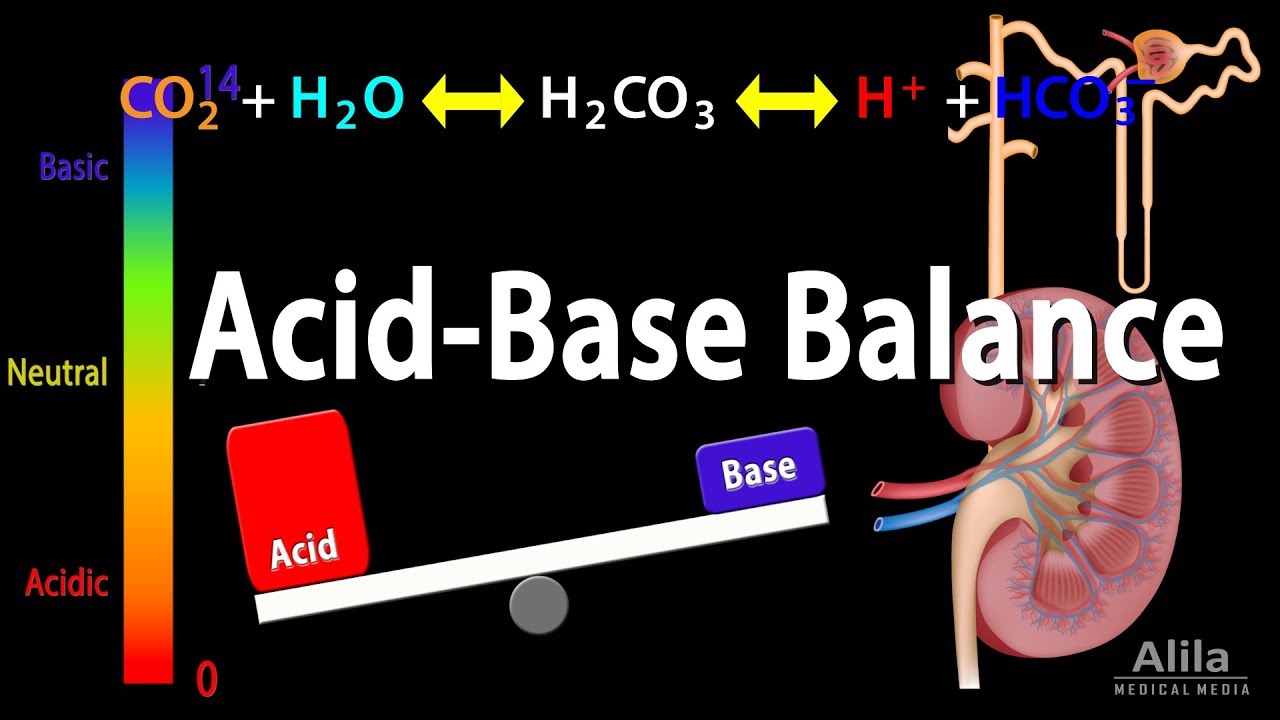
Image taken from the YouTube channel Alila Medical Media , from the video titled Acid Base Balance, Animation. .
Understanding a Primary Decrease in Plasma [H2CO3]
A "primary decrease in plasma [H2CO3]" (carbonic acid) signifies a reduction in the concentration of carbonic acid in the blood plasma. This can stem from a variety of underlying mechanisms and identifying these causes is crucial for appropriate treatment strategies. This document outlines the key causes contributing to this decrease and explores potential solutions.
Causes of Reduced Plasma [H2CO3]
A drop in plasma [H2CO3] isn’t often an isolated event, but a symptom of disturbances in the body’s acid-base balance. Several physiological processes influence carbonic acid levels.
Renal Dysfunction
The kidneys play a pivotal role in regulating bicarbonate (HCO3-) levels, which are closely linked to carbonic acid.
-
Renal Tubular Acidosis (RTA): This condition impairs the kidney’s ability to reabsorb bicarbonate or excrete acid. Different types of RTA exist, each impacting bicarbonate and therefore carbonic acid, differently.
- Proximal RTA (Type 2): Inability to reabsorb bicarbonate in the proximal tubule leads to significant bicarbonate wasting in the urine, reducing plasma bicarbonate and indirectly lowering carbonic acid.
- Distal RTA (Type 1): Impaired acid excretion in the distal tubule results in chronic metabolic acidosis, indirectly suppressing carbonic acid formation.
-
Chronic Kidney Disease (CKD): As kidney function declines overall, the ability to maintain acid-base balance is compromised, potentially leading to bicarbonate loss and reduced carbonic acid.
Respiratory Alkalosis
Respiratory alkalosis arises from hyperventilation, causing excessive carbon dioxide (CO2) exhalation. Since carbonic acid is formed from CO2 and water, a decrease in CO2 availability directly reduces carbonic acid levels.
-
Hyperventilation: This is the primary driver of respiratory alkalosis. Common causes include:
- Anxiety
- Pain
- Hypoxia (low oxygen levels)
- Certain medications
The relationship between CO2 and H2CO3 is described by the following simplified equation:
CO2 + H2O <–> H2CO3 <–> H+ + HCO3-
Decreasing CO2 drives the reaction to the left, decreasing H2CO3 concentration.
Metabolic Acidosis (Compensation)
While metabolic acidosis primarily involves a decrease in bicarbonate, the body attempts to compensate through respiratory mechanisms.
- Respiratory Compensation: In response to metabolic acidosis, the body increases respiratory rate to expel more CO2. This secondary reduction in CO2, as explained above, leads to a further reduction in carbonic acid. This is a compensatory mechanism, not a direct cause of the metabolic acidosis.
Medications
Certain medications can impact acid-base balance, leading to a decrease in plasma carbonic acid.
- Carbonic Anhydrase Inhibitors: These drugs (e.g., acetazolamide) inhibit the enzyme carbonic anhydrase, which catalyzes the conversion of CO2 and water to carbonic acid, and vice versa. By inhibiting this enzyme, the equilibrium is shifted, resulting in lower carbonic acid levels.
- Diuretics (Loop and Thiazide): Some diuretics can indirectly affect bicarbonate levels and thereby influence carbonic acid concentrations.
Solutions for Addressing Low Plasma [H2CO3]
Addressing a primary decrease in plasma [H2CO3] necessitates treating the underlying cause. Direct supplementation with carbonic acid is impractical due to its instability. The focus is on restoring proper acid-base balance.
Addressing Renal Dysfunction
Management depends on the specific type of renal dysfunction.
- RTA: Bicarbonate supplementation is often necessary to correct the acidosis. The specific dosage and form of bicarbonate (e.g., sodium bicarbonate, potassium citrate) depend on the RTA type and severity.
- CKD: Management includes controlling underlying conditions like hypertension and diabetes, dietary modifications (e.g., low protein), and, in advanced stages, dialysis or kidney transplant. Bicarbonate supplementation may be needed to counter metabolic acidosis.
Managing Respiratory Alkalosis
The primary goal is to address the underlying cause of hyperventilation.
- Anxiety: Therapy, relaxation techniques, and potentially medication.
- Pain: Pain management strategies.
- Hypoxia: Oxygen therapy.
- Medications: Adjusting or discontinuing causative medications.
Breathing techniques, such as paced breathing or breathing into a paper bag (though the use of paper bag breathing is now generally discouraged in many medical settings due to potential for worsening hypoxia in certain conditions), can also help to increase CO2 levels and subsequently carbonic acid.
Adjusting Medications
Careful review of medication lists is critical. If a medication is contributing to the acid-base imbalance, consider alternative therapies or dosage adjustments under the guidance of a healthcare professional.
| Medication Type | Mechanism of Action | Potential Impact on Plasma [H2CO3] |
|---|---|---|
| Carbonic Anhydrase Inhibitors | Inhibits carbonic anhydrase, reducing H2CO3 formation. | Direct decrease in plasma [H2CO3]. |
| Loop Diuretics | Affects electrolyte balance and renal bicarbonate handling. | Indirect impact; can lead to metabolic alkalosis or acidosis, affecting H2CO3 as a secondary consequence. |
| Thiazide Diuretics | Affects electrolyte balance and renal bicarbonate handling. | Indirect impact; can lead to metabolic alkalosis or acidosis, affecting H2CO3 as a secondary consequence. |
It is important to remember that this table provides a simplified overview. The actual effect of any medication depends on individual factors.
Plasma H2CO3 Drop: Your Questions Answered
Here are some frequently asked questions to help you better understand plasma H2CO3 drops and how to manage them.
What exactly does a "plasma H2CO3 drop" mean?
A plasma H2CO3 drop refers to a decrease in the level of bicarbonate (HCO3-) in your blood plasma. Bicarbonate is a crucial buffer that helps maintain the blood’s pH balance. A significant primary decrease in plasma [h2co3] is often indicative of underlying metabolic or respiratory issues.
What are the most common causes of a drop in plasma H2CO3?
Several factors can lead to a drop, including kidney problems (renal tubular acidosis), severe diarrhea, diabetic ketoacidosis (DKA), and certain medications. Excessive vomiting can also deplete bicarbonate levels. The primary decrease in plasma [h2co3] is usually a symptom of a larger health concern.
How is a low plasma H2CO3 level typically diagnosed?
Diagnosis starts with a blood test to measure your bicarbonate levels. Your doctor will also consider your medical history, symptoms, and potentially order other tests (like arterial blood gas analysis or kidney function tests) to pinpoint the underlying cause. A significant primary decrease in plasma [h2co3] warrants further investigation.
What are the potential treatments for a drop in plasma H2CO3?
Treatment depends entirely on the underlying cause. For example, DKA requires insulin and fluid replacement. In cases of renal tubular acidosis, bicarbonate supplementation may be necessary. Addressing the root cause is essential to restore normal plasma H2CO3 levels. The goal is to reverse the primary decrease in plasma [h2co3] by managing the underlying condition.
Alright, that’s the lowdown on what might be causing a primary decrease in plasma [h2co3]! Hopefully, this has cleared things up and given you some good pointers. Now go forth and conquer those metabolic mysteries!