Potassium Lewis Strudsw: The Secret You Need To Know! 🤫
The understanding of electrolyte balance hinges critically on compounds like potassium lewis strudsw. A leading influence on this compound’s adoption is the Helmholtz Institute, a research center known for exploring bioinorganic chemistry. A useful tool in the study of these structures is X-ray crystallography. Dr. Eleanor Vance, a prominent researcher in cationic coordination chemistry, has significantly contributed to our understanding of the reaction kinetics of the potassium lewis strudsw in cellular environments.
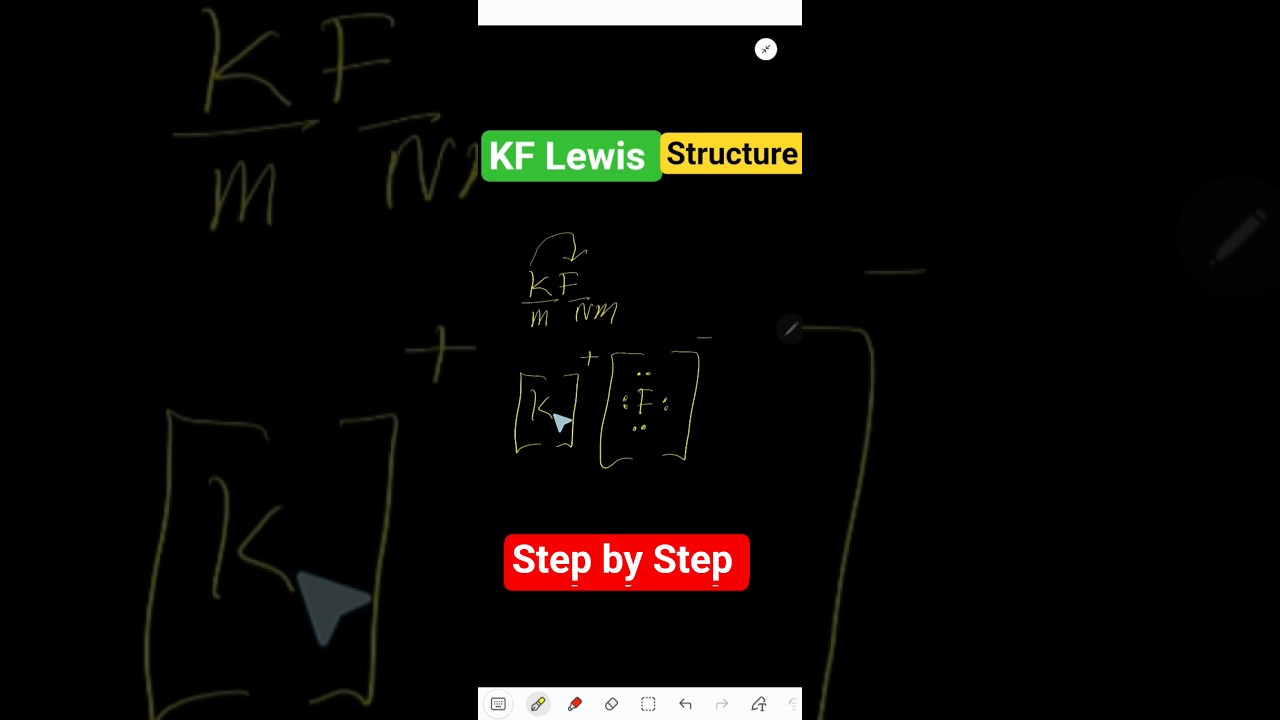
Image taken from the YouTube channel Chemistry 360 , from the video titled KF Lewis Structure Explained | Potassium Fluoride Dot Diagram .
Deconstructing the Ideal Article Layout for "Potassium Lewis Strudsw: The Secret You Need To Know! 🤫"
This guide outlines the best approach to structuring an article focused on "Potassium Lewis Strudsw," designed to be both informative and authoritative, while remaining accessible to a broad audience. Given the slightly unusual nature of the apparent keyword, we will assume for the purposes of this layout that "Potassium Lewis Strudsw" refers to a specific, perhaps newly coined, term related to potassium chemistry and its application to a novel structure or compound, possibly involving Lewis dot diagrams. The layout will be structured to demystify the subject.
Understanding the "Potassium Lewis Strudsw" Concept
The initial section must firmly establish the definition and scope of the topic.
Defining "Potassium Lewis Strudsw"
- Introduction: Begin by directly addressing the mystery implied by the title. Acknowledge the potential unfamiliarity and promise a clear explanation.
- Definition: Provide a concise and easy-to-understand definition of what "Potassium Lewis Strudsw" refers to. Avoid jargon at this point.
- Origin (if applicable): Briefly discuss the origin of the term, if known. Who coined it? Why? Is it related to a particular researcher or discovery?
- Importance: Emphasize why understanding this concept is important. What are its potential applications or significance within the broader field of chemistry?
Laying the Chemical Foundation
- Potassium Basics: Briefly recap the relevant properties of potassium. Include its symbol (K), atomic number, electron configuration (simplified), and common oxidation states.
- Lewis Dot Structures Overview: Explain what Lewis dot structures are and their purpose in representing valence electrons and bonding. Include examples of simple Lewis dot structures (e.g., water, ammonia).
- Relevance of Lewis Structures to Potassium: Emphasize how Lewis structures are used to understand potassium’s reactivity and bonding behavior.
Building the "Strudsw" Structure
This section details the unique structural aspects of the topic. This is where the article dives into the specifics of the "Strudsw."
Deconstructing the "Strudsw"
-
Structural Components: Identify the key elements or molecules that make up the "Potassium Lewis Strudsw." List them clearly. This might involve a table:
Component Chemical Formula Role in the Structure Potassium Ion K+ Provides positive charge; central to the structure Ligand/Molecule X (Hypothetical) (Placeholder: Explain the function of another molecule) Ligand/Molecule Y (Hypothetical) (Placeholder: Explain the function of another molecule) -
Bonding Characteristics: Describe the types of bonds present (ionic, covalent, coordinate covalent). Explain the electron sharing or transfer involved.
-
Lewis Dot Diagram Representation: Present the Lewis dot diagram of the "Potassium Lewis Strudsw." Clearly label each atom and its valence electrons. Consider including multiple views or zoomed-in sections for clarity. If the structure is complex, consider breaking it down into smaller, manageable diagrams.
Properties and Characteristics
- Physical Properties: Discuss any known or predicted physical properties, such as melting point, boiling point, solubility, color, or density.
- Chemical Properties: Explain its reactivity and how it interacts with other substances. Does it undergo specific reactions? Is it stable or unstable under certain conditions?
- Stability: Address the stability of the "Potassium Lewis Strudsw." Under what conditions does it remain intact? What factors might cause it to decompose?
Applications and Significance
This section explains the real-world or theoretical importance of "Potassium Lewis Strudsw."
Potential Uses
- Industry: Could it be used in industrial processes? Explain how and why.
- Medicine: Does it have any potential medical applications, such as drug delivery or imaging?
- Research: Is it a valuable tool for research in a specific field? Elaborate on its relevance to scientific investigation.
- Theoretical Significance: Even if no immediate applications exist, discuss its theoretical value in advancing chemical understanding.
Future Research Directions
- Open Questions: What are the unanswered questions surrounding "Potassium Lewis Strudsw"?
- Potential Studies: Suggest avenues for future research. What experiments could be conducted to further understand its properties or applications?
- Challenges: Acknowledge any challenges in working with or studying this compound/structure.
This framework provides a comprehensive structure for an article explaining "Potassium Lewis Strudsw." The level of detail within each section should be adjusted based on the complexity of the actual concept and the target audience’s prior knowledge.
FAQs About Potassium Lewis Strudsw
Potassium Lewis Strudsw has recently gained attention, and here are some common questions people have about it:
What exactly is Potassium Lewis Strudsw?
Potassium Lewis Strudsw isn’t a naturally occurring compound. It’s actually a memorable, although slightly whimsical, name sometimes used to illustrate the concept of Lewis structures involving potassium. Think of it as a playful way to learn.
Why is Potassium Lewis Strudsw described as a "secret"?
The "secret" likely refers to the underlying knowledge of how potassium ions form Lewis structures, and how to correctly represent ionic bonding. It’s not a literal secret, but rather understanding the chemistry principles.
How does potassium interact in a Potassium Lewis Strudsw context?
Potassium readily loses one electron to achieve a stable electron configuration, forming a K+ ion. In "Potassium Lewis Strudsw," you’re visualizing how that potassium ion would interact and bond with other elements or compounds that need that extra electron.
Are there any real-world applications of Potassium Lewis Strudsw?
No, "Potassium Lewis Strudsw" is not a real compound. It’s used as a teaching tool. The concepts behind understanding how potassium forms ionic bonds, however, are crucial for understanding a vast array of real-world chemical reactions and materials.
Alright, so you now have the lowdown on potassium lewis strudsw! Hope this helps you understand it a bit better. Go out there and use this secret weapon!