Is Isopropanol Polar? The SHOCKING Truth Revealed!
The understanding of molecular structure fundamentally impacts chemical reactions, and this principle directly relates to the polarity of isopropanol. The National Institute of Standards and Technology (NIST) provides crucial data regarding the properties of solvents, enabling accurate predictions about their behavior. Further analysis using tools like Computational Chemistry software can help elucidate why the polarity of isopropanol influences its effectiveness as a disinfectant.
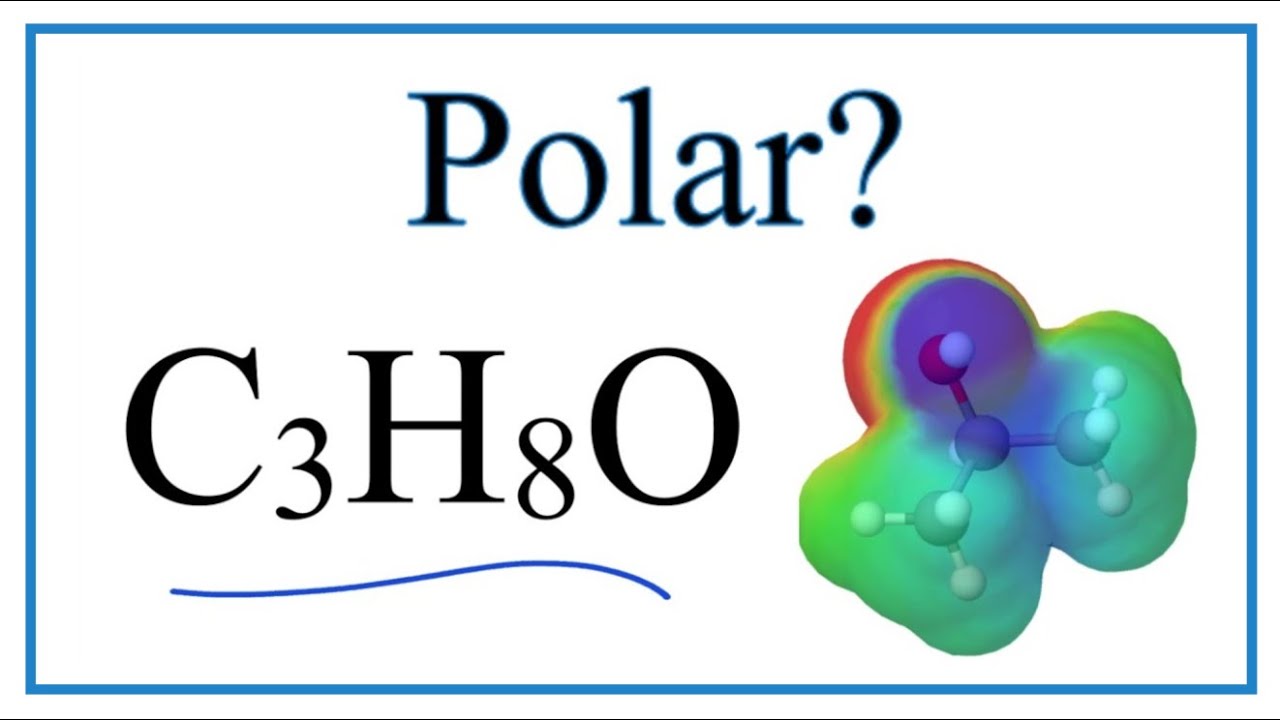
Image taken from the YouTube channel Wayne Breslyn (Dr. B.) , from the video titled Is C3H8O Polar on Non-Polar (Isopropyl alcohol or 2-Propanol) .
Unveiling the Polarity of Isopropanol: A Comprehensive Guide
This article aims to thoroughly explore the polarity of isopropanol, a common solvent, revealing the contributing factors and dispelling potential misconceptions. The focus will be on providing a clear and easily understandable explanation of the "polarity of isopropanol."
Understanding Polarity: A Foundation
Before delving into isopropanol specifically, it’s crucial to establish a basic understanding of polarity in molecules.
What Makes a Molecule Polar?
Polarity arises from unequal sharing of electrons within a molecule. This unequal sharing occurs when atoms with differing electronegativities form a chemical bond. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond.
- Electronegativity Difference: The greater the difference in electronegativity between two bonded atoms, the more polar the bond.
- Dipole Moment: This difference in electronegativity creates a dipole moment, representing the magnitude and direction of the polarity within the bond. We can visualize this with an arrow pointing towards the more electronegative atom.
- Molecular Geometry: Crucially, individual bond dipoles do not always result in an overall polar molecule. The molecule’s geometry plays a significant role. If the bond dipoles cancel each other out due to symmetry, the molecule will be nonpolar.
How to Measure Polarity: Dielectric Constant
While we can infer polarity based on molecular structure, a practical measure is the dielectric constant.
- Definition: The dielectric constant indicates a substance’s ability to reduce the electric field between two capacitor plates. A higher dielectric constant means the substance is more polar.
- Relevance: It provides a quantitative measure of polarity useful in comparing different solvents. Water has a high dielectric constant (around 80), indicating high polarity, whereas hexane has a low dielectric constant (around 2) indicating very low polarity.
Analyzing the Structure of Isopropanol
Isopropanol, also known as isopropyl alcohol or 2-propanol, has the chemical formula CH3CH(OH)CH3. Understanding its structure is paramount to understanding its polarity.
Chemical Formula and Structural Representation
The structure reveals the presence of hydroxyl (-OH) group bonded to the central carbon atom. This is the key feature contributing to its polarity.
Electronegativity Considerations
The oxygen atom in the -OH group is significantly more electronegative than both carbon and hydrogen.
- O-H Bond Polarity: This electronegativity difference creates a strong dipole moment in the O-H bond, with oxygen pulling electron density towards itself.
- C-O Bond Polarity: Similarly, the carbon-oxygen bond also exhibits polarity, although to a lesser extent than the O-H bond.
Delving Deeper: Is Isopropanol Polar?
Based on the structural analysis, isopropanol is indeed polar. However, it’s not as polar as water.
The Polar Hydroxyl Group
The presence of the hydroxyl group is the dominant factor causing isopropanol’s polarity. As explained before, the significant electronegativity difference between oxygen and hydrogen leads to a partial negative charge on the oxygen atom and partial positive charges on the hydrogen and carbon atoms.
The Nonpolar Alkyl Groups
Isopropanol also contains two methyl groups (CH3) attached to the central carbon atom. These alkyl groups are relatively nonpolar.
- C-H Bond Polarity: The electronegativity difference between carbon and hydrogen is small, leading to a relatively weak dipole moment in the C-H bonds.
- Overall Contribution: The presence of these nonpolar alkyl groups reduces the overall polarity of the isopropanol molecule compared to a molecule like water, which contains only highly polar O-H bonds.
Polarity Compromise: Amphiphilic Nature
Due to the presence of both polar and nonpolar regions, isopropanol exhibits amphiphilic properties, meaning it has an affinity for both polar and nonpolar substances.
Comparing Isopropanol’s Polarity to Other Solvents
To get a better sense of where isopropanol stands on the polarity spectrum, it’s helpful to compare it to other common solvents.
| Solvent | Dielectric Constant (Approximate) | Polarity |
|---|---|---|
| Water | 80 | High |
| Isopropanol | 18 | Intermediate |
| Ethanol | 24 | Intermediate |
| Acetone | 21 | Intermediate |
| Hexane | 2 | Low |
- Water (High): Very polar due to the two O-H bonds.
- Ethanol (Intermediate): Similar to isopropanol, with a hydroxyl group but a slightly different alkyl chain, affecting its polarity subtly.
- Acetone (Intermediate): Polar due to the carbonyl group (C=O), which has a significant dipole moment.
- Hexane (Low): Nonpolar due to the presence of only C-H and C-C bonds, with minimal electronegativity differences.
The table clearly shows that isopropanol falls in the intermediate range, between highly polar solvents like water and nonpolar solvents like hexane. This intermediate polarity makes isopropanol a versatile solvent in various applications.
FAQs: Is Isopropanol Polar? The SHOCKING Truth Revealed!
These frequently asked questions will further clarify the polarity of isopropanol and its implications.
Why is isopropanol considered polar despite having a nonpolar section?
Isopropanol’s polarity arises from the presence of the hydroxyl (-OH) group. While the isopropyl (CH3)2CH- portion is nonpolar, the -OH group’s strong electronegativity difference between oxygen and hydrogen creates a dipole moment, making the overall molecule polar, albeit less polar than water. This allows isopropanol to dissolve both polar and nonpolar substances.
How does the polarity of isopropanol affect its uses?
The polarity of isopropanol allows it to be an effective solvent. It can dissolve a wider range of substances compared to purely nonpolar solvents. This is why it’s used as a cleaning agent, disinfectant, and in various industrial processes to dissolve both polar and nonpolar contaminants.
Is isopropanol more or less polar than water?
Isopropanol is less polar than water. Water has two highly polar O-H bonds creating a larger net dipole moment. The larger nonpolar section of isopropanol dampens its overall polarity, making it less polar than water but still significantly polar enough to dissolve polar substances.
How does the polarity of isopropanol compare to other alcohols?
The polarity of alcohols generally decreases as the alkyl chain length increases. Methanol (one carbon) is more polar than ethanol (two carbons), which is more polar than isopropanol (three carbons). This trend is because the nonpolar alkyl chain becomes more dominant, reducing the overall polarity of the molecule despite the presence of the -OH group.
So, now you know the deal with polarity of isopropanol! Hopefully, this clears things up for you. Feel free to experiment and explore more – chemistry is all about discovery!