Oxygen Bohr Model: The Ultimate Guide You Need to Read!
The Bohr Model, a foundational concept in atomic physics, provides a simplified yet insightful representation of atomic structure. Understanding how this model applies to oxygen, an element vital for life and combustion, requires a grasp of quantum numbers. Ernest Rutherford’s contributions to atomic theory paved the way for Bohr’s model, highlighting the significance of a central nucleus. Exploring the oxygen bohr model reveals not only the electron configuration of oxygen but also its influence on chemical bonding and molecular properties.
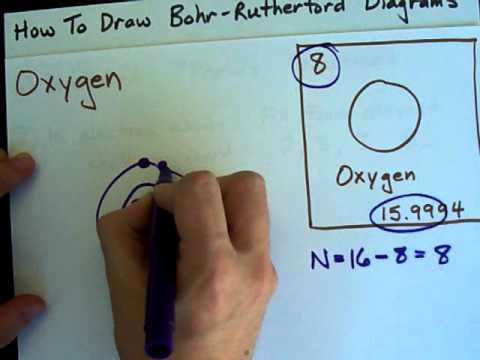
Image taken from the YouTube channel chemistNATE , from the video titled How to Draw Bohr-Rutherford Diagrams – Oxygen .
Oxygen Bohr Model: The Ultimate Guide – Article Layout
This outlines an effective article layout for explaining the "Oxygen Bohr Model". The aim is clarity, thoroughness, and accessibility, making it easy for readers to understand the topic, regardless of their prior knowledge.
Introduction: Setting the Stage
- Hook: Start with a compelling hook that immediately grabs the reader’s attention. This could be a surprising fact about oxygen, or a common misconception about atomic models. Example: "Did you know that understanding how oxygen atoms behave is crucial for breathing and combustion? The Bohr model helps us visualize that!"
- What is the Bohr Model?: Briefly define the Bohr model in general terms. Explain that it is a simplified representation of atomic structure. Note its limitations but also its value as a learning tool.
- Why Oxygen?: Explain why focusing on oxygen is important. Oxygen is essential for life, relevant to various scientific disciplines, and a good example for illustrating the Bohr model’s principles.
- Article Roadmap: Briefly outline what the article will cover. This helps the reader understand the structure and anticipate the content.
Fundamentals: Atomic Structure Refresher
- What is an Atom?: Define an atom as the basic building block of matter.
- Atomic Number and Mass Number: Define these terms and explain their significance. The atomic number of oxygen is 8, and its mass number is approximately 16 (for the most common isotope).
- Protons, Neutrons, and Electrons: Briefly explain each subatomic particle and their role in the atom.
- Oxygen’s Subatomic Particles: State explicitly the number of protons, neutrons, and electrons in a neutral oxygen atom. (8 protons, 8 neutrons, 8 electrons)
- Electron Shells (Energy Levels): Introduce the concept of electron shells or energy levels. Explain that electrons orbit the nucleus in specific energy levels.
The Bohr Model for Oxygen: A Step-by-Step Explanation
-
Visual Representation: Include a clear diagram of the oxygen Bohr model. The diagram should show the nucleus with the correct number of protons and neutrons, and the electron shells with the electrons arranged correctly. Use color-coding to distinguish the particles.
-
Electron Configuration: Explain the electron configuration of oxygen.
- First Shell (K Shell): Describe that the first shell can hold a maximum of 2 electrons and that oxygen has 2 electrons in its first shell.
- Second Shell (L Shell): Explain that the second shell can hold a maximum of 8 electrons and that oxygen has 6 electrons in its second shell.
- Visual Representation: Illustrate this with a point form:
- Shell 1: 2 electrons
- Shell 2: 6 electrons
-
Valence Electrons: Define valence electrons as the electrons in the outermost shell. Explain that oxygen has 6 valence electrons.
- Importance of Valence Electrons: Explain that valence electrons determine the chemical properties of an element and how it forms bonds with other atoms.
-
Stability and the Octet Rule: Explain the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a full outer shell with 8 electrons (except for hydrogen and helium, which aim for 2). Explain that oxygen is most stable when it has 8 electrons in its outer shell. This explains why it bonds with other atoms.
Limitations of the Bohr Model
- Simplification: Emphasize that the Bohr model is a simplified representation and does not accurately depict the true complexity of atomic structure.
- Wave-Particle Duality: Explain that electrons behave as both particles and waves, a concept not captured by the Bohr model.
- Modern Atomic Theory: Briefly mention that quantum mechanics provides a more accurate and complete description of atomic structure, including the concept of electron orbitals (instead of fixed orbits).
Applications and Relevance
- Understanding Chemical Bonding: Explain how the Bohr model can help visualize how oxygen forms chemical bonds with other elements (e.g., in water (H₂O) or carbon dioxide (CO₂)).
- Predicting Reactivity: Discuss how the number of valence electrons can predict the reactivity of oxygen.
- Examples of Oxygen-Containing Molecules: Provide several examples of common oxygen-containing molecules and explain their significance (e.g., water, carbon dioxide, ozone).
Comparison Table: Bohr Model vs. Quantum Mechanical Model
Present a table that directly compares and contrasts the Bohr model with the quantum mechanical model of the atom.
| Feature | Bohr Model | Quantum Mechanical Model |
|---|---|---|
| Electron Orbit | Fixed, circular orbits | Orbitals (probability distributions) |
| Electron Energy | Quantized energy levels | Quantized energy levels |
| Wave-Particle Duality | Not considered | Accounts for wave-particle duality |
| Accuracy | Less accurate | More accurate |
| Complexity | Simpler | More complex |
Interactive Elements (Optional, enhances engagement)
- Quiz: Include a short quiz to test the reader’s understanding of the material.
- Interactive Diagram: Embed an interactive diagram of the oxygen Bohr model that allows users to zoom in and explore the different parts of the atom.
- Simulation: Include a simulation that shows how oxygen atoms form bonds with other atoms.
FAQs: Understanding the Oxygen Bohr Model
Have questions about the oxygen Bohr model? This FAQ addresses some common points to help solidify your understanding.
What exactly does the Bohr model for oxygen show?
The oxygen Bohr model is a simplified representation of an oxygen atom, specifically how its electrons are arranged in energy levels or shells around the nucleus. It illustrates the number of protons and neutrons in the nucleus and the distribution of electrons in distinct orbits.
How does the oxygen Bohr model differ from reality?
The oxygen Bohr model is a simplified model. In reality, electrons don’t orbit the nucleus in neat, circular paths like planets. Instead, they exist in probability regions called orbitals. The model is a useful teaching tool but not a precise representation.
How many electrons are in the outermost shell of an oxygen atom according to the Bohr model?
The oxygen Bohr model shows that oxygen has 6 electrons in its outermost shell, also known as its valence shell. This is crucial for understanding oxygen’s reactivity and how it forms chemical bonds with other elements.
Why is understanding the oxygen Bohr model important?
Understanding the oxygen Bohr model provides a fundamental grasp of atomic structure and electron configuration. It’s a building block for learning about chemical bonding, molecular properties, and the behavior of oxygen in chemical reactions. It provides a good introductory understanding of the element.
So, there you have it! We hope this deep dive into the oxygen bohr model helped clear things up. Now go forth and use this knowledge – you’ve got this!