Nitrogen’s Electron Structure: Master It Now! (Explained)
Understanding nitrogen electron structure is fundamental to grasping the behavior of numerous chemical compounds. Linus Pauling’s work significantly contributed to our understanding of chemical bonding, a concept intricately linked to nitrogen’s electron arrangement. Spectroscopy serves as a powerful tool for analyzing and confirming the predicted electronic configuration of nitrogen. Moreover, the Haber-Bosch process, vital for ammonia production, relies heavily on manipulating nitrogen electron structure to facilitate efficient reactions. Ultimately, mastering nitrogen electron structure unlocks a deeper comprehension of chemical processes in diverse fields.
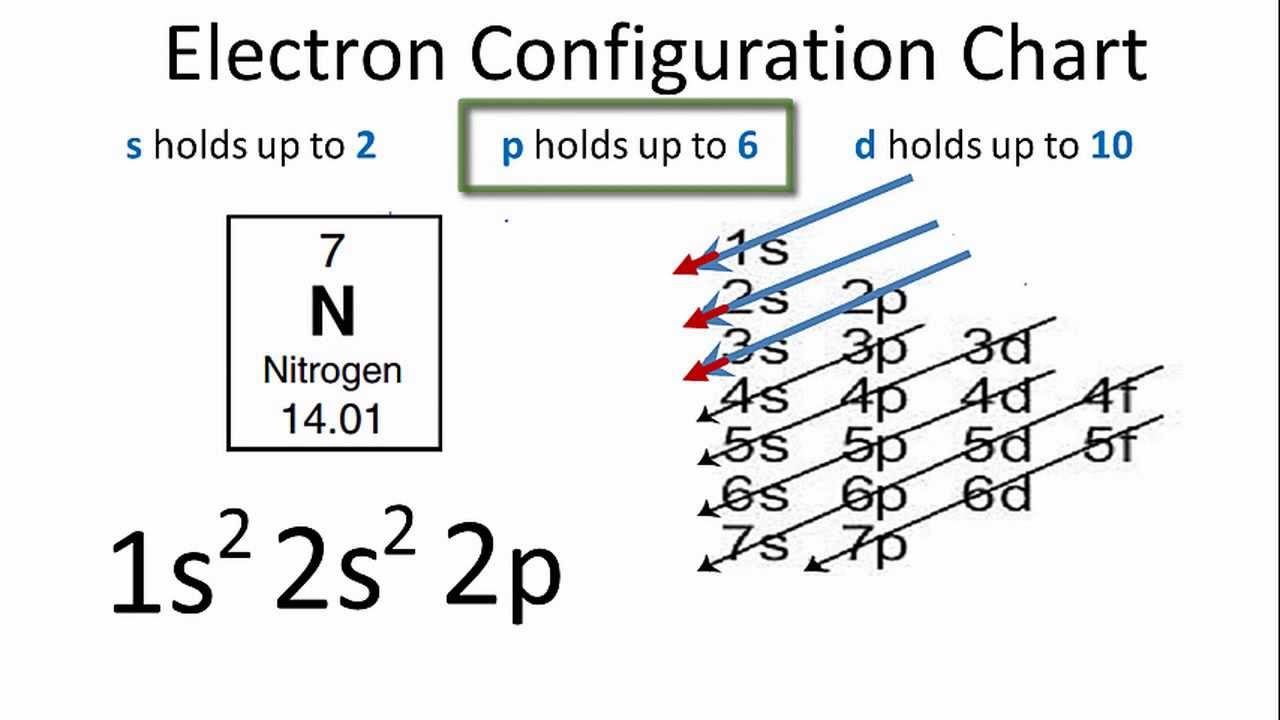
Image taken from the YouTube channel Wayne Breslyn (Dr. B.) , from the video titled Nitrogen Electron Configuration .
Decoding Nitrogen’s Electron Structure: A Step-by-Step Guide
Understanding the electron structure of nitrogen is key to grasping its chemical behavior. This article breaks down the often-confusing topic into manageable steps, making it easier to master the concept. Our focus is on providing a clear and accessible explanation of "nitrogen electron structure".
1. Foundational Concepts: Atoms and Electrons
Before diving directly into nitrogen, it’s helpful to recap some essential atomic theory.
1.1. The Atomic Model
Atoms, the basic building blocks of matter, comprise a central nucleus surrounded by orbiting electrons. The nucleus contains positively charged protons and neutral neutrons. Electrons are negatively charged and exist in distinct energy levels or "shells" around the nucleus.
1.2. Electron Shells and Orbitals
- Shells: These are like concentric orbits around the nucleus, each corresponding to a specific energy level. The first shell (closest to the nucleus) can hold a maximum of 2 electrons. The second shell can hold up to 8, and so on.
- Orbitals: Within each shell, electrons reside in regions called orbitals. These are described by specific shapes and orientations. s orbitals are spherical, p orbitals are dumbbell-shaped, and so on. Each orbital can hold a maximum of 2 electrons, with opposite spins.
2. Understanding Nitrogen: Atomic Number and Electron Configuration
Nitrogen (symbol N) has some key characteristics that influence its electron structure.
2.1. Atomic Number
Nitrogen’s atomic number is 7. This means each nitrogen atom has 7 protons in its nucleus and, in its neutral state, 7 electrons orbiting the nucleus.
2.2. Electron Configuration: A Notation Guide
Electron configuration describes the arrangement of electrons in the different shells and orbitals of an atom. We use a specific notation for this:
- The number indicates the energy level (shell number).
- The letter indicates the type of orbital (s, p, d, f).
- The superscript number indicates the number of electrons in that orbital.
For example, 1s² means the s orbital in the first shell has 2 electrons.
3. Unveiling Nitrogen’s Electron Structure
Now, let’s apply the concepts to nitrogen.
3.1. Filling the Electron Shells
Nitrogen has 7 electrons. Following the rules:
- The first shell (n=1) fills first. It can hold a maximum of 2 electrons. Therefore, we have 1s².
- This leaves us with 5 electrons to place. These go into the second shell (n=2).
- The second shell has s and p orbitals. The s orbital fills first, accommodating 2 electrons: 2s².
- Finally, the remaining 3 electrons go into the p orbitals: 2p³.
3.2. The Complete Electron Configuration of Nitrogen
Putting it all together, the electron configuration of nitrogen is: 1s² 2s² 2p³.
3.3. Visualizing Nitrogen’s Electron Arrangement
We can represent this visually in a variety of ways:
-
Orbital Diagram: This diagram shows the distribution of electrons within individual orbitals, including their spin. Each orbital is represented by a box, and each electron by an arrow (pointing up or down to indicate spin).
1s: ↑↓
2s: ↑↓
2p: ↑ ↑ ↑Notice that in the 2p orbitals, the 3 electrons occupy each orbital individually before pairing up (Hund’s rule).
-
Simplified Shell Diagram: This diagram shows the number of electrons in each shell.
Shell Electrons 1 (K) 2 2 (L) 5
4. Importance of Nitrogen’s Electron Structure
Understanding the arrangement of electrons in nitrogen is crucial for predicting its chemical behavior.
4.1. Valence Electrons and Bonding
The electrons in the outermost shell (valence electrons) are the ones involved in chemical bonding. Nitrogen has 5 valence electrons (2 in the 2s orbital and 3 in the 2p orbitals). This explains why nitrogen often forms three covalent bonds to achieve a stable octet (8 electrons) in its valence shell.
4.2. Examples of Nitrogen Bonding
- Ammonia (NH₃): Nitrogen shares three electrons with three hydrogen atoms, forming three single covalent bonds.
- Nitrogen Gas (N₂): Two nitrogen atoms share three pairs of electrons, forming a strong triple bond. This is why nitrogen gas is relatively inert.
By understanding the electron structure of nitrogen, you can begin to predict how it will interact with other elements and form compounds.
Frequently Asked Questions: Nitrogen’s Electron Structure
Understanding the nitrogen electron structure is essential for grasping its chemical behavior. Here are some common questions addressed:
Why does nitrogen form three bonds?
Nitrogen has five valence electrons. To achieve a stable octet (eight electrons), it needs to gain three more. This is why nitrogen readily forms three covalent bonds, sharing electrons with other atoms. This leads to the strong triple bond seen in molecular nitrogen (N₂).
What is the electron configuration of nitrogen?
The electron configuration of nitrogen is 1s² 2s² 2p³. This shows that nitrogen has two electrons in its 1s orbital, two in its 2s orbital, and three in its 2p orbitals. These valence electrons determine the nitrogen electron structure.
What are the implications of nitrogen’s electron configuration for its reactivity?
The electron configuration, particularly the presence of three unpaired p-electrons, makes nitrogen relatively reactive. It readily forms compounds with other elements to complete its octet. However, the strong triple bond in N₂ requires significant energy to break, making atmospheric nitrogen relatively inert.
How does the nitrogen electron structure relate to its electronegativity?
Nitrogen is a relatively electronegative element. This means it has a strong tendency to attract electrons in a chemical bond. This electronegativity stems from the nitrogen electron structure and its need to gain electrons to achieve a stable electron configuration. This makes it a key player in polar covalent bonds.
So, there you have it! We hope you’ve gained a better handle on nitrogen electron structure. Keep exploring, keep experimenting, and most importantly, keep learning! You’ve got this!