Lithium Balmer Series: Demystifying Atomic Spectra (Explained)
The lithium balmer series, an atomic emission spectra phenomenon, provides significant insights into quantum mechanics. Spectroscopy, the analytical technique used to observe this series, facilitates understanding of atomic energy levels within lithium atoms. Niels Bohr’s atomic model serves as a foundational framework for interpreting observed spectral lines. Furthermore, research conducted at the National Institute of Standards and Technology (NIST) contributes substantially to the precise measurement and characterization of lithium balmer series emissions.
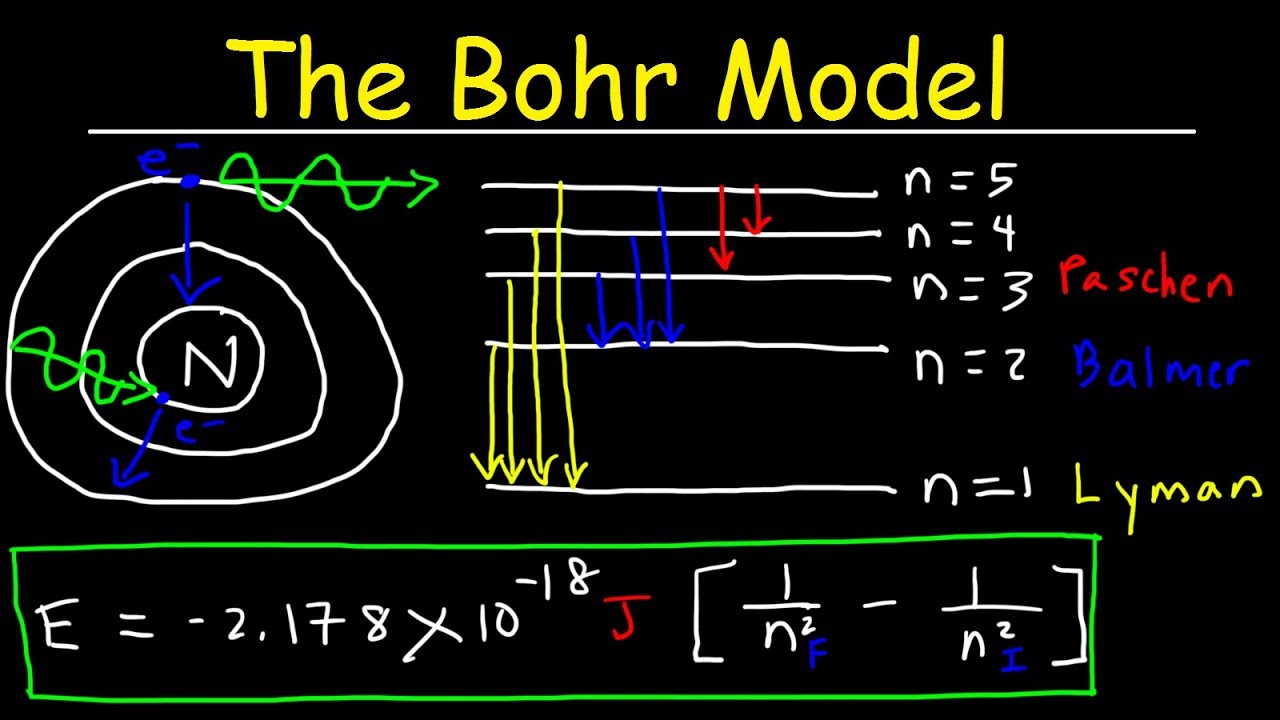
Image taken from the YouTube channel The Organic Chemistry Tutor , from the video titled Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer Series .
Lithium Balmer Series: Article Layout Explanation
A successful article on the "Lithium Balmer Series" needs a logical structure that builds understanding step-by-step. This outline details the best layout for explaining this specific atomic spectra topic.
Introduction: Setting the Stage
The introduction must immediately establish the context and significance of the topic.
- Hook: Begin with a captivating question or a real-world example connected to atomic spectroscopy or lithium’s uses (e.g., in batteries, medicine).
- Brief Overview: Summarize what the Balmer series is in general terms, without diving into specifics. Emphasize that it’s a series of spectral lines emitted when an electron transitions between energy levels in an atom.
- Focus on Lithium: Clearly state that the article will focus on the Balmer series specifically for lithium, highlighting any unique characteristics it may possess compared to hydrogen.
- Purpose Statement: Clearly state the goal of the article: to demystify the lithium Balmer series and explain its significance.
- Keyword Integration: Seamlessly incorporate the primary keyword "lithium Balmer series" within the introduction, preferably in the first paragraph, and throughout the introduction.
Understanding Atomic Spectra
This section lays the groundwork for understanding the Balmer series by explaining atomic spectra in general.
What are Atomic Spectra?
- Explanation: Define atomic spectra as the unique pattern of light emitted or absorbed by an element.
- Emission vs. Absorption Spectra: Explain the difference between emission and absorption spectra, using clear visual aids (e.g., diagrams showing electron transitions). Briefly mention how these spectra act like "fingerprints" for elements.
- Quantization of Energy: This is crucial. Explain that electrons can only exist at specific energy levels within an atom. Avoid complex quantum mechanics; use simple analogies like climbing steps (each step is a specific energy level).
The Role of Electron Transitions
- Energy Level Diagram: Include a clear energy level diagram for a generic atom (not necessarily lithium yet). Show the electron transitions that result in the emission or absorption of photons.
- Photon Emission: Explain that when an electron transitions from a higher energy level to a lower energy level, it emits a photon of light. The energy of the photon is equal to the difference in energy between the two levels.
- Photon Absorption: Explain the reverse process, where an electron absorbs a photon of specific energy to jump to a higher energy level.
- Relationship Between Energy, Wavelength, and Frequency: Introduce the equations that connect these properties:
- E = hf (Energy = Planck’s constant * frequency)
- c = fλ (Speed of light = frequency * wavelength)
- E = hc/λ (Energy = Planck’s constant * speed of light / wavelength)
- Explain what each variable represents. Keep the math simple.
The Balmer Series Explained
This section introduces the concept of the Balmer series as applied to all atoms, laying the foundation before focusing specifically on lithium.
Defining the Balmer Series
- Transition to n=2: Explain that the Balmer series corresponds to electron transitions that end at the n=2 energy level (the second energy level) in an atom.
- Visible Light Region: Emphasize that transitions to n=2 typically produce photons in the visible light region of the electromagnetic spectrum.
- Hydrogen Balmer Series: Briefly mention the hydrogen Balmer series as the most well-known and simplest example. Explain why hydrogen’s simple electronic structure makes its spectra easier to analyze.
The Rydberg Formula
-
Introducing the Formula: Present the Rydberg formula, which predicts the wavelengths of spectral lines in the Balmer series:
1/λ = R (1/2² – 1/n²)
Where:
- λ is the wavelength of the emitted photon
- R is the Rydberg constant (approximately 1.097 x 10⁷ m⁻¹)
- n is an integer greater than 2 (n = 3, 4, 5, …)
-
Explanation of Variables: Clearly define each variable in the formula.
-
Calculating Wavelengths: Show a simple example calculation using the Rydberg formula to predict the wavelength of one of the hydrogen Balmer lines (e.g., n=3 to n=2).
The Lithium Balmer Series: Specifics
Now, we focus on the primary topic: the Balmer series for lithium.
Lithium’s Electronic Structure
- Atomic Number and Electronic Configuration: State the atomic number of lithium (3) and its electronic configuration (1s²2s¹).
- Valence Electron: Emphasize the single valence electron (2s¹), which is responsible for the majority of its optical properties.
- Why Lithium is Different: Explain that while lithium is similar to hydrogen in having a single valence electron, the presence of the core electrons (1s²) introduces complexities. These core electrons shield the valence electron from the full nuclear charge, affecting energy levels and the resulting spectra.
Wavelengths of the Lithium Balmer Series
-
Empirical Data vs. Theoretical Prediction: Discuss the challenges in accurately predicting the lithium Balmer series wavelengths solely from theoretical calculations due to electron-electron interactions.
-
Table of Key Lithium Balmer Lines: Provide a table listing the major lithium Balmer lines, along with their experimentally determined wavelengths and corresponding transitions (e.g., 2p → 2s, 3s → 2p, 3d → 2p). Be sure to indicate the transitions involving p and d orbitals, as these are relevant to lithium.
Transition Wavelength (nm) 2p → 2s 670.78 3d → 2p [Value] 3s → 2p [Value] … … -
Hyperfine Structure (If Applicable): If appropriate for the target audience, briefly touch upon the hyperfine structure of the lithium Balmer lines due to nuclear spin. Acknowledge that this adds complexity, but avoid in-depth explanations if it is beyond the article’s scope.
Applications of the Lithium Balmer Series
- Spectroscopic Analysis: Explain how the lithium Balmer series can be used to identify lithium in various samples.
- Plasma Diagnostics: Briefly mention its use in determining plasma parameters (e.g., temperature, density) in fusion research or other plasma applications.
- Atomic Clocks: If relevant, mention the use of specific lithium transitions (not necessarily Balmer series lines, but related) in atomic clocks.
Tools and Techniques for Observing Lithium Balmer Series
- Spectrometers: Briefly describe the role of spectrometers in observing and analyzing atomic spectra.
- Experimental Setup: Provide a simple diagram or description of a typical experimental setup used to measure the lithium Balmer series. This could involve a lithium light source, a spectrometer, and a detector.
- Data Analysis: Explain how the data obtained from the spectrometer is analyzed to identify the specific Balmer lines and measure their wavelengths.
FAQs: Lithium Balmer Series Explained
Here are some frequently asked questions to further clarify the Lithium Balmer Series and its significance in atomic spectra.
What exactly is the Lithium Balmer Series?
The lithium Balmer series refers to a specific set of spectral lines emitted by lithium atoms when electrons transition from higher energy levels down to the n=2 energy level. These transitions release energy in the form of photons, creating the visible lines observed in the spectrum. However, due to lithium’s atomic structure, its Balmer series isn’t as prominent or straightforward as hydrogen’s.
Why isn’t the Lithium Balmer Series as easily observed as Hydrogen’s?
Lithium has more than one electron, unlike hydrogen. This means electron interactions and shielding effects complicate the energy levels and transitions. In effect, it influences the wavelengths and intensities of the emitted light, making the lithium Balmer series less distinct compared to hydrogen’s.
What information can we gain from studying the Lithium Balmer Series?
Analyzing the lithium Balmer series, despite its complexities, provides valuable insights into lithium’s atomic structure and energy levels. By studying the wavelengths and intensities of the spectral lines, scientists can refine atomic models and understand the interactions between electrons in lithium.
Are there any practical applications for studying the Lithium Balmer Series?
While not as widely used as other elements’ spectral series, the study of the lithium Balmer series contributes to a deeper understanding of atomic physics and spectroscopy. This knowledge is crucial for fields such as astrophysics (studying the composition of stars) and plasma physics (analyzing the behavior of ionized gases).
Hopefully, this deep dive made the lithium balmer series a bit clearer! Go forth and explore the fascinating world of atomic spectra. Happy experimenting!