Chlorine’s Electronic Structure: Explained Simply!
Understanding the electronic structure of elements is fundamental to chemistry. Quantum mechanics provides the theoretical framework for describing this structure, which directly impacts an element’s behavior and bonding properties. The element chlorine (Cl), known for its uses in water treatment and sanitation, serves as an excellent example for illustrating these principles. A vital concept here is electron configuration, the distribution of electrons within the different energy levels and sublevels of an atom. This explanation will help you understand that a chlorine atom has 17 electrons what are its electronic structure in numbers, including the influence of the Aufbau principle in determining its electron configuration.
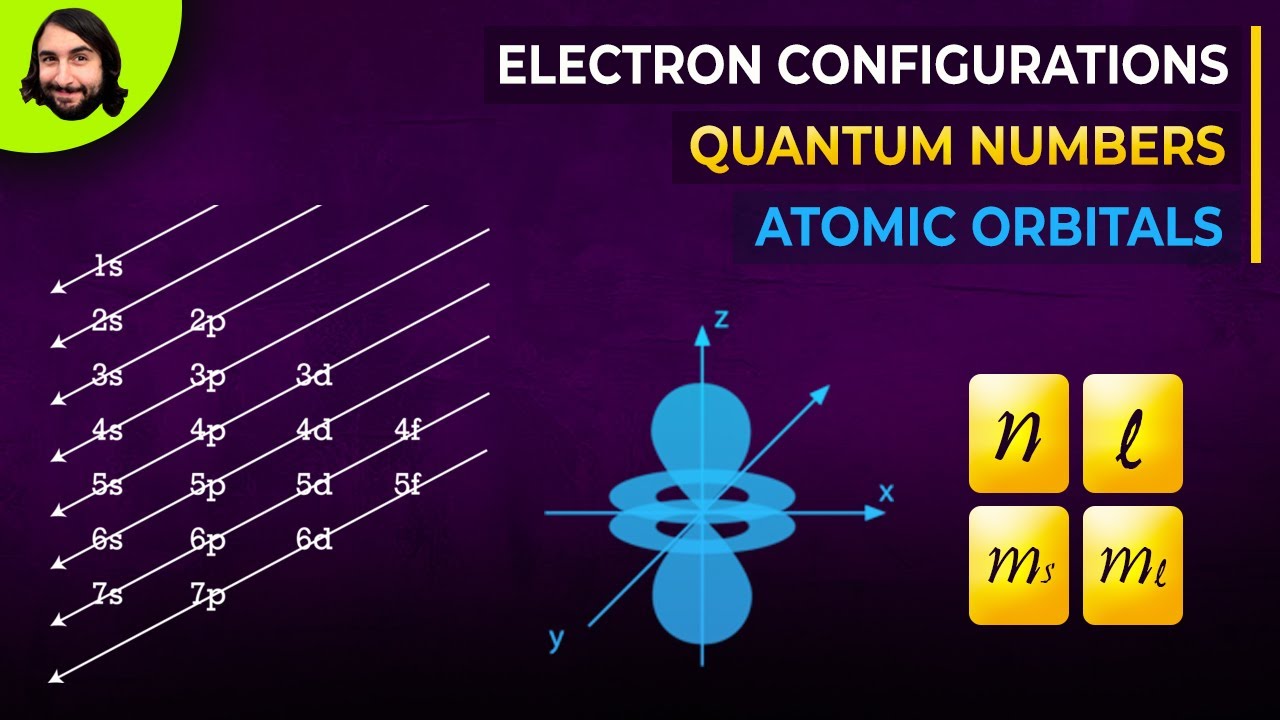
Image taken from the YouTube channel Professor Dave Explains , from the video titled Quantum Numbers, Atomic Orbitals, and Electron Configurations .
Chlorine (Cl), a ubiquitous element in our daily lives, often plays an unsung, yet crucial role. From disinfecting our drinking water and swimming pools to serving as a vital building block in the chemical industry, chlorine’s presence is both pervasive and impactful.
But what exactly makes chlorine so reactive and essential? The answer lies within its atomic structure, specifically the arrangement of its electrons.
Decoding the Electronic Enigma
This article aims to demystify the electron configuration of chlorine, presenting it in a clear and accessible manner for a general audience. We’ll break down the seemingly complex arrangement of electrons into digestible concepts, shedding light on why chlorine behaves the way it does.
No prior in-depth knowledge of chemistry is required. We’ll build from the ground up, ensuring that everyone can understand this fundamental aspect of chemistry.
The Atomic Count: 17 Electrons
A chlorine atom is characterized by the presence of 17 electrons. These electrons are not randomly scattered. They are meticulously organized around the atom’s nucleus in specific energy levels and orbitals.
Understanding how these 17 electrons are arranged is the key to unlocking chlorine’s chemical behavior. This arrangement governs its ability to form bonds, react with other elements, and ultimately, defines its role in the world around us. By exploring its electron configuration, we begin to unravel the secrets of this vital element.
Decoding the electronic structure of chlorine requires a foundational understanding of how electrons are organized within an atom. Before diving into the specifics of chlorine’s configuration, it’s essential to establish the basic principles that govern electron arrangement. These principles provide the framework for understanding why chlorine possesses its unique chemical properties.
Atomic Number and Electron Arrangement: Laying the Groundwork
At the heart of every element lies its atomic number, a fundamental identifier that dictates its very essence. This number isn’t arbitrary; it represents the number of protons nestled within the atom’s nucleus.
The Atomic Number’s Role
For an electrically neutral atom—and chlorine is no exception in its elemental state—the number of protons equals the number of electrons.
Therefore, knowing that chlorine’s atomic number is 17 immediately tells us that a neutral chlorine atom possesses 17 electrons. This equality is crucial for maintaining electrical balance.
A General Overview of Electron Arrangement
These 17 electrons don’t simply float around randomly. They exist in a highly structured arrangement around the nucleus, governed by the principles of quantum mechanics.
Imagine the atom as a miniature solar system. The nucleus, containing protons and neutrons, sits at the center, much like the sun. The electrons orbit this nucleus, not in fixed paths, but in specific energy levels, often visualized as shells.
The electrons closest to the nucleus are held most tightly, possessing the lowest energy. Electrons farther out have higher energy and are less tightly bound.
Introducing Electron Shells and Energy Levels
These energy levels are also referred to as electron shells. Each shell can accommodate a specific number of electrons. The first shell (closest to the nucleus) can hold a maximum of 2 electrons; the second, a maximum of 8; and the third, up to 18 (though the rules for filling become more complex for larger atoms).
This shell structure dictates the overall electronic configuration. For chlorine with its 17 electrons, the first two shells are filled, and the remaining electrons begin to populate the third shell.
Understanding this basic shell model is the first step in deciphering the more detailed electron configuration of chlorine.
The Periodic Table as a Guide
The Periodic Table serves as a powerful tool for predicting and understanding electronic structure. Elements within the same group (vertical column) share similar chemical properties. This similarity arises from having the same number of electrons in their outermost shell, known as valence electrons.
Chlorine resides in Group 17, the halogens. This placement indicates that chlorine has 7 valence electrons, a characteristic shared by other halogens like fluorine and bromine. The Periodic Table, therefore, provides an invaluable shortcut to understanding electronic configurations and predicting chemical behavior.
Electron Shells and Subshells: Diving into the Details
We’ve established that electrons reside in distinct energy levels around the nucleus, often visualized as shells. But the organization of these electrons is even more nuanced than simply existing in a particular shell. Each shell itself is further divided into subshells, adding another layer of complexity to the electronic architecture of an atom.
Understanding Electron Shells (n = 1, 2, 3…)
Electron shells are designated by the principal quantum number, ‘n,’ which can be any positive integer (1, 2, 3, and so on). These numbers correspond to the energy level of the shell.
The higher the value of ‘n,’ the greater the energy and the farther the shell is from the nucleus.
Think of them as floors in a building, with the ground floor (n=1) being the lowest energy and closest to the foundation (the nucleus).
- n = 1 (The K Shell): This innermost shell is closest to the nucleus and can hold a maximum of two electrons.
- n = 2 (The L Shell): The second shell can accommodate up to eight electrons.
- n = 3 (The M Shell): The third shell can hold up to 18 electrons.
And so on.
Populating the Shells: Energy Level Dictates Order
Electrons fill these shells in a specific order, dictated by the principle of minimizing energy. Electrons will always occupy the lowest available energy level first.
This means the innermost shell (n=1) fills before the second shell (n=2), and so on.
However, within each shell, the subshells also have slightly different energy levels, influencing the filling order even further.
Introducing Subshells (s, p, d, f) and Atomic Orbitals
Each electron shell is composed of one or more subshells, denoted by the letters s, p, d, and f. These subshells represent regions of space within a shell where electrons are most likely to be found.
Each subshell contains one or more atomic orbitals. An atomic orbital is a mathematical function that describes the wave-like behavior of an electron in an atom. Each orbital can hold a maximum of two electrons, according to the Pauli Exclusion Principle.
- s subshells: Each shell contains one s subshell, which consists of a single, spherical orbital (holding a max of 2 electrons).
- p subshells: Starting with the second shell (n=2), each shell contains a p subshell, composed of three dumbbell-shaped orbitals oriented along the x, y, and z axes (holding a max of 6 electrons).
- d subshells: The third shell (n=3) introduces the d subshell, which has five orbitals with more complex shapes (holding a max of 10 electrons).
- f subshells: Finally, the fourth shell (n=4) includes the f subshell, comprising seven orbitals with even more intricate shapes (holding a max of 14 electrons).
The number of orbitals in a subshell directly dictates the maximum number of electrons it can hold. Understanding these subshells and their orbitals is vital to deciphering the specific arrangement of electrons within an atom.
Chlorine’s Electron Configuration: A Step-by-Step Breakdown
Having explored the architecture of electron shells and subshells, we can now pinpoint the specific arrangement of electrons within a chlorine atom. This arrangement, or electron configuration, dictates how chlorine interacts with other atoms, ultimately defining its chemical behavior.
Decoding Chlorine’s Electronic Signature: 1s² 2s² 2p⁶ 3s² 3p⁵
The electron configuration of chlorine is represented as 1s² 2s² 2p⁶ 3s² 3p⁵. Let’s break down this notation:
-
The numbers (1, 2, 3) represent the electron shells (n=1, n=2, n=3).
-
The letters (s, p) denote the subshells within each shell.
-
The superscripts indicate the number of electrons occupying each subshell.
Therefore, chlorine has two electrons in the 1s subshell, two electrons in the 2s subshell, six electrons in the 2p subshell, two electrons in the 3s subshell, and five electrons in the 3p subshell.
This distribution accounts for all 17 electrons of the chlorine atom.
The Significance of the 3p⁵ Configuration: A Quest for Stability
Notice that the outermost shell (n=3) contains two subshells: 3s and 3p. The 3s subshell is completely filled with two electrons (3s²). However, the 3p subshell contains only five electrons (3p⁵), whereas it can hold a maximum of six.
This incomplete 3p subshell is the key to understanding chlorine’s high reactivity.
Atoms strive for stability, which is achieved when their outermost electron shell is completely filled (the octet rule, with some exceptions).
Chlorine, with its nearly full 3p subshell, has a strong tendency to gain one more electron to complete its octet.
This eagerness to acquire an electron makes chlorine a highly reactive element, particularly with elements that readily donate electrons.
Valence Electrons and Chemical Bonding: The Outermost Players
The electrons in the outermost shell, also known as the valence shell, are called valence electrons.
These are the electrons that participate in chemical bonding.
For chlorine, the valence shell is the third shell (n=3). It contains seven valence electrons: two in the 3s subshell and five in the 3p subshell.
This means chlorine can form a single covalent bond by sharing one electron with another atom or form an ionic bond by gaining one electron from another atom.
Chlorine’s seven valence electrons dictate its ability to form strong bonds, making it a crucial component in countless chemical compounds.
Chlorine and the Periodic Table: A Family Affair
Chlorine’s quest for a complete octet doesn’t occur in isolation. Its chemical tendencies, and indeed its electron configuration, are intimately linked to its location on the periodic table.
Understanding its placement unlocks a deeper appreciation for why chlorine behaves the way it does and how it relates to other elements.
Decoding Group 17: The Halogen Family
Chlorine resides in Group 17 of the periodic table, also known as the halogens. This group is characterized by elements with seven valence electrons – that is, seven electrons in their outermost shell.
This shared electronic characteristic is not coincidental; it’s the very reason they’re grouped together.
Other prominent halogens include fluorine (F), bromine (Br), and iodine (I), each with similar electron configurations, albeit with increasing numbers of electron shells.
The similarities in their outer electron arrangement dictate their shared chemical behaviors.
The Electron Configuration-Position Nexus
The periodic table isn’t just a random arrangement of elements. It is organized to reflect the electronic structure of atoms.
Elements in the same group share similar valence electron configurations, leading to similar chemical properties.
Chlorine’s [Ne] 3s² 3p⁵ configuration is a hallmark of its group. The [Ne] represents the electron configuration of Neon, the noble gas that precedes chlorine in the periodic table.
Each halogen, in turn, exhibits a valence electron configuration of ns² np⁵, where n represents the outermost electron shell.
Fluorine, for example, has the configuration 1s² 2s² 2p⁵, while bromine boasts [Ar] 4s² 3d¹⁰ 4p⁵.
The ns² np⁵ pattern explains their shared eagerness to gain a single electron to achieve a stable octet.
Electronegativity and Shared Traits
One of the most significant properties shared by halogens is their high electronegativity. Electronegativity describes an atom’s ability to attract electrons in a chemical bond.
Halogens, with their near-complete outer shells, exert a strong pull on electrons. Chlorine, in particular, is highly electronegative, second only to fluorine.
This high electronegativity makes halogens potent oxidizing agents, readily accepting electrons from other substances.
This tendency is also responsible for their corrosive nature and their ability to form strong ionic bonds with electropositive elements such as alkali metals (Group 1).
Trends Down the Group
While halogens share key characteristics, there are also trends that manifest as we move down the group from fluorine to iodine.
Electronegativity decreases, and atomic size increases.
Fluorine is the most reactive halogen, while iodine is the least. The larger atomic size of iodine also leads to weaker attraction for electrons.
Even with these variations, the fundamental drive to gain one electron and achieve a stable octet remains a defining feature of the halogen family, firmly rooted in their electron configurations and position within the periodic table.
The elegance and predictability of chlorine’s electron configuration might suggest a simple, almost predetermined arrangement. However, to truly appreciate the ‘why’ behind the 1s² 2s² 2p⁶ 3s² 3p⁵ sequence, we must acknowledge the underlying framework governing the quantum realm. It is here, in the domain of quantum mechanics, that the true foundations of electronic structure are revealed.
Quantum Mechanics: The Guiding Hand
While we can describe and predict electron configurations using rules and patterns, it’s crucial to understand that these rules are not arbitrary. They emerge from the fundamental laws of physics governing the behavior of matter at the atomic level.
Quantum mechanics, a branch of physics developed in the early 20th century, provides the framework for understanding how electrons behave within atoms.
It’s a complex and often counterintuitive theory, but its principles dictate the allowed energy levels and spatial distributions of electrons around the nucleus.
In essence, quantum mechanics tells us that electrons can only exist in specific, quantized energy states.
These states correspond to the electron shells and subshells we’ve discussed, meaning an electron can’t just have any energy; it must occupy a defined energy level.
Wave-Particle Duality and Probability
One of the core tenets of quantum mechanics is the concept of wave-particle duality.
This means that electrons, despite being considered particles, also exhibit wave-like properties.
This wave-like behavior is described by mathematical functions called wavefunctions, the squares of which give the probability of finding an electron in a particular region of space.
This isn’t to say that electron locations are totally random, but rather they exist in a probabilistic cloud around the nucleus.
It is why we often visualize atomic orbitals as probability distributions rather than fixed paths.
The Schrödinger Equation: A Quantum Recipe
At the heart of quantum mechanics lies the Schrödinger equation.
This equation, while mathematically complex, acts as a recipe for determining the allowed energy levels and wavefunctions of electrons in an atom.
Solving the Schrödinger equation for a given atom provides information about the energies and spatial distributions of its electrons.
This is how physicists and chemists predict electron configurations from first principles.
While solving the Schrödinger equation for multi-electron atoms like chlorine is computationally challenging, the underlying principle remains the same: the electronic structure is a direct consequence of the quantum mechanical laws governing the behavior of electrons.
No Arbitrary Arrangements
Therefore, chlorine’s electron configuration is not simply a matter of convention or convenience; it’s a consequence of the physical laws that govern the universe at the smallest scales. The seemingly simple arrangement of electrons is deeply rooted in the principles of quantum mechanics. These principles dictate the allowed energy levels, shapes, and spatial orientations of electron orbitals.
By understanding that quantum mechanics is the bedrock upon which electron configurations are built, we gain a deeper appreciation for the elegance and order that underlies the seemingly complex world of atomic structure. Although delving into the mathematical intricacies of quantum mechanics is beyond the scope of this discussion, it is crucial to acknowledge its central role in shaping the electronic identity of chlorine and all other elements.
FAQs: Understanding Chlorine’s Electronic Structure
Here are some frequently asked questions to help you further understand the electronic structure of chlorine.
What does "electronic structure" actually mean?
Electronic structure refers to how electrons are arranged within an atom. It describes which energy levels (or shells) and sublevels (or orbitals) the electrons occupy. Since a chlorine atom has 17 electrons what are its electronic structure in numbers, understanding its electronic structure is vital.
How are the electrons in a chlorine atom arranged?
Chlorine’s 17 electrons are arranged in three electron shells. The first shell holds 2 electrons, the second shell holds 8 electrons, and the outermost (valence) shell holds 7 electrons. This can be represented as 2, 8, 7.
Why is the outermost shell so important?
The outermost shell, also known as the valence shell, is crucial because it determines how chlorine interacts with other atoms to form chemical bonds. The seven valence electrons of chlorine make it highly reactive.
What is the full electron configuration notation for chlorine?
The full electron configuration notation for chlorine is 1s² 2s² 2p⁶ 3s² 3p⁵. This notation shows how the 17 electrons in a chlorine atom has 17 electrons what are its electronic structure in numbers are distributed among the different orbitals within each energy level.
So, now you’ve got a grip on how those 17 electrons are arranged in a chlorine atom! Hopefully, understanding a chlorine atom has 17 electrons what are its electronic structure in numbers makes a little more sense now. Keep exploring the fascinating world of chemistry!