Decoding Fluorine: The Bohr Model Explained Simply!
The periodic table, a cornerstone of chemistry, arranges elements like fluorine based on their atomic structure. Niels Bohr’s pivotal work on atomic structure offers a framework for understanding this arrangement. Understanding the electron configuration of fluorine helps chemists understand how it bonds with other elements. This article, therefore, aims to simplify decoding fluorine’s electronic structure through the lens of the bohr model fluourine. The resulting models give a visualization for chemists and students of quantum mechanics.
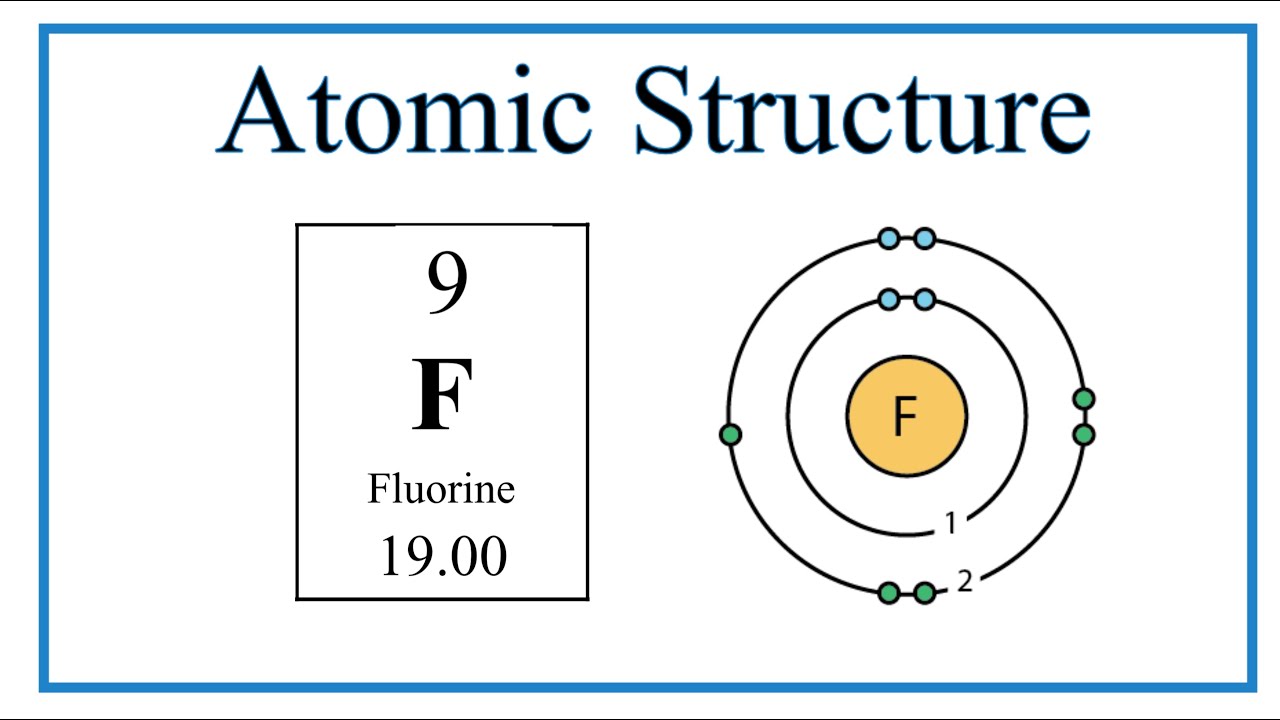
Image taken from the YouTube channel Wayne Breslyn (Dr. B.) , from the video titled Atomic Structure (Bohr Model) for Fluorine (F .
Fluorine, a pale yellow gas, holds a prominent position in the periodic table, not just for its unique properties but also for its exceptional reactivity. This eagerness to bond with almost anything makes it both incredibly useful and potentially dangerous. From strengthening our teeth in toothpaste to playing a critical role in pharmaceuticals and materials science, fluorine’s influence is undeniable.
But what lies at the heart of this remarkable reactivity? The answer, of course, resides within its atomic structure.
This article aims to peel back the layers of fluorine’s atomic architecture, offering a clear understanding of its fundamental composition. We will explore fluorine’s structure through the lens of the Bohr model, a simplified yet powerful tool for visualizing the atom.
Our goal is to illustrate how the arrangement of electrons within the fluorine atom dictates its behavior, revealing why it is such an active participant in the chemical world.
Fluorine: The Reactive Powerhouse
Fluorine (chemical symbol F) is the most electronegative element, meaning it has an unparalleled ability to attract electrons to itself. This stems from its electron configuration. Its atomic structure is characterized by a nearly complete outer electron shell. This electron arrangement drives its intense reactivity.
This reactivity makes fluorine a vital component in various industrial processes and consumer products. It is used in the production of Teflon (non-stick cookware) and certain medications, as well as being added to municipal water supplies and dental products to prevent tooth decay.
The element’s properties also present challenges. Fluorine gas is corrosive and toxic, requiring careful handling and storage. Its compounds can have significant environmental impacts, requiring stringent regulations for their use and disposal.
Deciphering the Atom: A Glimpse into Atomic Structure
Before diving into the specifics of fluorine, let’s take a moment to review the basics of atomic structure. Atoms, the fundamental building blocks of all matter, are composed of three primary particles: protons, neutrons, and electrons.
-
Protons are positively charged particles located in the atom’s nucleus.
-
Neutrons, also residing in the nucleus, have no charge (they are neutral).
-
Electrons are negatively charged particles that orbit the nucleus in specific energy levels or shells.
The number of protons in an atom defines its atomic number and, consequently, its identity as a specific element. For example, all atoms with one proton are hydrogen, all atoms with six protons are carbon, and so on. The number of electrons typically equals the number of protons in a neutral atom, ensuring a balanced charge. The arrangement and behavior of these electrons dictate how an atom interacts with other atoms, forming molecules and compounds.
Fluorine’s remarkable reactivity, as we’ve seen, is deeply intertwined with its atomic composition. But to truly grasp the nuances of this relationship, we need a framework for understanding the atom itself. This is where the Bohr model enters the picture, providing a foundational, albeit simplified, view of atomic structure.
The Bohr Model: A Stepping Stone to Atomic Understanding
The Bohr model, proposed by Niels Bohr in 1913, represents a pivotal moment in the history of atomic theory.
It was a revolutionary departure from earlier models that struggled to explain the stability of atoms and the discrete nature of atomic spectra.
While superseded by more sophisticated quantum mechanical models, the Bohr model remains invaluable for its intuitive clarity and its ability to introduce fundamental concepts.
A Historical Perspective
Prior to Bohr, the prevailing model, often likened to a "planetary model," envisioned electrons orbiting the nucleus in a manner similar to planets orbiting the sun.
However, classical physics predicted that such orbiting electrons would continuously radiate energy, spiraling into the nucleus and causing the atom to collapse.
Bohr’s genius lay in incorporating the revolutionary ideas of quantum theory, pioneered by Max Planck, to resolve this paradox.
His model provided a stable and quantifiable picture of the atom that resonated with the scientific community and paved the way for further advancements.
Key Postulates of the Bohr Model
The Bohr model is based on several key postulates that fundamentally changed our understanding of atomic structure:
- Quantized Energy Levels: Electrons can only occupy specific energy levels, often visualized as distinct orbits around the nucleus. These energy levels are quantized, meaning that electrons can only possess certain discrete amounts of energy.
- Electron Orbits: Electrons orbit the nucleus in these defined energy levels, without radiating energy. Each orbit corresponds to a specific energy state of the electron.
- Quantum Leaps: Electrons can transition between energy levels by absorbing or emitting energy in the form of photons (light). The energy of the photon is equal to the difference in energy between the two levels.
These postulates, though simplified, provided a framework for understanding the stability of atoms and the emission of light at specific wavelengths.
The model successfully predicted the spectrum of hydrogen, a significant achievement that solidified its place in the history of physics.
Niels Bohr: The Architect of Atomic Structure
Niels Bohr’s contribution to atomic physics is immeasurable. His model provided the first successful explanation of atomic spectra and laid the groundwork for quantum mechanics.
Bohr received the Nobel Prize in Physics in 1922 for his work on atomic structure.
Beyond the specific details of his model, Bohr’s emphasis on quantization and the role of energy levels has had a lasting impact on our understanding of the atomic world.
His work serves as a powerful example of how theoretical models, even those with limitations, can drive scientific progress and unlock new frontiers of knowledge.
Fluorine’s remarkable reactivity, as we’ve seen, is deeply intertwined with its atomic composition. But to truly grasp the nuances of this relationship, we need a framework for understanding the atom itself. This is where the Bohr model enters the picture, providing a foundational, albeit simplified, view of atomic structure.
Fluorine’s Atomic Architecture: A Bohr Model Perspective
The Bohr model offers a clear lens through which to examine the architecture of the fluorine atom. By understanding fluorine’s atomic number, electron configuration, and electron shell arrangement within the context of the Bohr model, we can start to unravel the secrets of its chemical behavior.
Unveiling Fluorine’s Identity: The Atomic Number
Fluorine’s atomic number is 9. This seemingly simple number is the key to understanding fluorine’s fundamental identity. The atomic number defines the number of protons found within the nucleus of every fluorine atom.
It’s this proton count that irrevocably distinguishes fluorine from all other elements. Change the number of protons, and you change the element itself.
The presence of nine positively charged protons dictates that, in a neutral fluorine atom, there must also be nine negatively charged electrons to balance the charge. This balance is crucial for the atom’s overall stability and its ability to interact with other atoms.
Deciphering the Electron Configuration: 1s²2s²2p⁵
The electron configuration of fluorine, denoted as 1s²2s²2p⁵, provides a roadmap for how these nine electrons are arranged around the nucleus according to the Bohr model’s principles. Each part of this notation reveals critical information about the distribution of electrons within different energy levels and sublevels.
The "1s²" indicates that two electrons occupy the innermost electron shell, also known as the K shell. This shell is closest to the nucleus and represents the lowest energy level.
The "2s²2p⁵" tells us about the second electron shell, or the L shell. This shell can accommodate up to eight electrons, but in fluorine, it holds a total of seven: two in the 2s subshell and five in the 2p subshell.
This electron configuration is critical because it reveals that fluorine has seven valence electrons – electrons in the outermost shell that participate in chemical bonding. This near-complete outer shell is the driving force behind fluorine’s exceptional reactivity, as it readily seeks to gain one more electron to achieve a stable octet configuration.
Visualizing Electron Shells: K, L, and Beyond
Imagine the fluorine atom as a miniature solar system. At the center lies the nucleus, containing nine protons and, typically, ten neutrons (for the most common isotope, Fluorine-19). Orbiting this nucleus are the electrons, arranged in distinct shells or energy levels.
The innermost shell, the K shell, can hold a maximum of two electrons. In fluorine, this shell is completely filled.
The next shell, the L shell, can accommodate up to eight electrons. Fluorine’s L shell contains seven electrons, leaving it just one electron short of being completely full.
The Bohr model, while a simplification, provides a powerful visual representation of these electron shells and their importance in determining an element’s chemical properties. Although the Bohr Model does not explicitly account for M shell in Fluorine, we can infer based on the filling principle.
Deciphering the electron configuration unveils the arrangement of electrons.
It’s a static snapshot, but the reality is far more dynamic.
Electrons aren’t simply fixed in place. They exist within specific energy levels.
But before we dive deeper into these electron dynamics, let’s take a step back and consider the fundamental forces at play.
Energy Levels and Fluorine’s Electron Dynamics
In the Bohr model, electrons orbit the nucleus at distinct energy levels, often visualized as concentric shells.
These aren’t arbitrary locations. They are quantized states.
This means electrons can only possess specific amounts of energy, corresponding to these defined orbits.
Quantized Energy Levels in Fluorine
Fluorine, with its nine electrons, exemplifies this principle.
The first two electrons fill the innermost shell (K-shell), which has the lowest energy level.
These are the 1s² electrons in the configuration.
The remaining seven electrons occupy the second shell (L-shell).
This shell is comprised of the 2s² and 2p⁵ electrons.
It’s crucial to recognize that electrons don’t reside between these energy levels.
They can only exist at them, and must absorb or emit energy to transition from one level to another.
This quantized nature is a cornerstone of atomic behavior.
The Significance of Valence Electrons
The outermost electron shell, also known as the valence shell, holds particular significance.
The electrons residing in this shell are called valence electrons.
These are the key players in chemical reactions.
Fluorine has seven valence electrons (2s²2p⁵).
This nearly complete outer shell makes it highly reactive.
Fluorine readily seeks to gain one more electron to achieve a stable octet configuration.
Achieving this noble gas configuration drives its propensity to form chemical bonds.
Fluorine’s Thirst for Electrons
This "thirst" for an electron explains fluorine’s exceptional electronegativity, its ability to attract electrons in a chemical bond.
It’s the driving force behind many of its reactions.
The vigorous reactions of fluorine highlight this principle in action.
It aggressively extracts electrons from other elements to complete its octet.
A Quantum Mechanical Perspective
While the Bohr model provides a valuable foundation, it’s essential to acknowledge its limitations.
Quantum mechanics offers a more accurate and nuanced description of energy levels.
Instead of fixed orbits, electrons exist in probability distributions called orbitals.
These orbitals represent regions where electrons are most likely to be found.
Furthermore, quantum mechanics introduces the concept of subshells (s, p, d, f) within each energy level, providing a more detailed picture of electron distribution.
While the Bohr model simplifies this picture, it still captures the essence of quantized energy levels that dictate an atom’s chemical behavior.
The Indelible Mark of Protons
While electron configuration dictates reactivity, the identity of an atom is defined by the number of protons in its nucleus.
Fluorine, by definition, possesses nine protons.
Change that number, and you change the element entirely.
The number of protons dictates the positive charge of the nucleus, which, in turn, governs the number of electrons an atom will have in its neutral state.
This balance between protons and electrons is fundamental to the atom’s stability and its interactions with other atoms.
Fluorine readily seeks to gain that single electron to achieve a stable octet configuration. However, while the Bohr model provides a valuable foundation for understanding these concepts, it’s essential to recognize its limitations, paving the way for a deeper dive into the realm of modern atomic theory.
Beyond Bohr: Limitations and Modern Atomic Theory
The Bohr model, with its neat electron orbits, offers a simplified, almost classical picture of the atom. It’s an incredibly useful tool for initial understanding, particularly for elements with fewer electrons. However, its depiction of atomic structure, especially for elements like fluorine with its more complex electron interactions, falls short of accurately representing the nuanced reality.
The Bohr Model’s Inadequacies
One of the primary shortcomings lies in its treatment of electrons as particles orbiting the nucleus in fixed paths. This is a significant oversimplification. It violates the principles of quantum mechanics, which dictate that electrons exhibit wave-particle duality.
Furthermore, the Bohr model struggles to explain the spectra of atoms more complex than hydrogen. The predicted spectral lines often deviate from experimental observations. The model simply cannot account for the subtle interactions between multiple electrons in larger atoms.
Another limitation is its inability to accurately predict chemical bonding and molecular geometry. The rigid orbits of the Bohr model don’t allow for the flexibility and dynamic interactions observed in real chemical bonds.
The Rise of Quantum Mechanics
To overcome these limitations, the world of physics turned to quantum mechanics, a more sophisticated and accurate framework for describing atomic behavior. Quantum mechanics abandons the concept of fixed electron orbits in favor of atomic orbitals.
These orbitals are mathematical functions that describe the probability of finding an electron in a particular region of space around the nucleus. Unlike the defined paths of the Bohr model, quantum mechanics depicts electrons as existing in a cloud of probability.
Quantum Mechanical View of Fluorine
From a quantum mechanical perspective, the electron configuration of fluorine (1s²2s²2p⁵) takes on a richer meaning. The 1s, 2s, and 2p notations represent specific atomic orbitals with distinct shapes and energy levels.
The two electrons in the 1s orbital occupy the region closest to the nucleus. The two electrons in the 2s orbital are slightly further out, while the five electrons in the 2p orbitals are distributed along three perpendicular axes.
These orbitals are not fixed in space. Instead, they represent the probability distributions of electron density. The electrons are most likely to be found in these regions, but they can also exist elsewhere, albeit with a lower probability.
The Impact of Quantum Mechanics
The advent of quantum mechanics revolutionized our understanding of atomic structure and chemical bonding. It provides a more accurate and comprehensive description of electron behavior. It allows us to predict the properties of molecules with greater precision.
Quantum mechanics also underpins many of the technologies we rely on today, from lasers and semiconductors to magnetic resonance imaging (MRI). Understanding its principles is crucial for advancing scientific knowledge and technological innovation.
Decoding Fluorine: Your Bohr Model Questions Answered
Want to understand how Fluorine works according to the Bohr Model? Here are some of the most commonly asked questions:
How many electron shells does Fluorine have according to the Bohr model?
Based on the bohr model, fluorine has two electron shells. The first shell holds a maximum of two electrons, and the second shell in fluorine contains seven electrons.
What determines Fluorine’s chemical properties based on the Bohr model fluorine?
According to the Bohr model, the electrons in the outermost shell, also known as valence electrons, mainly define the properties of Fluorine. Fluorine has seven valence electrons. It needs only one more to complete its octet.
How does the Bohr model help visualize Fluorine’s electron arrangement?
The Bohr model fluorine visually represents Fluorine’s electrons orbiting the nucleus in defined paths or shells. This makes it easier to understand how Fluorine’s electrons are arranged and how they potentially form bonds.
Is the Bohr model a completely accurate depiction of Fluorine’s electron structure?
While useful for visualizing the basic concept of electron arrangement, the Bohr model is a simplified view. More advanced models offer a more accurate depiction of the atom’s electron behavior, but the Bohr model serves as a great entry point.
So there you have it – a simple breakdown of the bohr model fluorine! Hopefully, now you’ve got a better handle on how it all works. Keep exploring and good luck with your chemistry adventures!