BCC Lattice Meaning Explained: The Ultimate Guide!
The crystal structure plays a vital role in materials science, and understanding it requires exploring various lattice arrangements. Body-centered cubic (BCC) structure represents one such arrangement, exhibiting characteristics that influence material properties. The National Institute of Standards and Technology (NIST) provides data and standards crucial for accurate characterization of materials with BCC lattices. Therefore, a comprehensive understanding of bcc lattice meaning is foundational for engineers and scientists utilizing techniques like X-ray diffraction to analyze material composition.
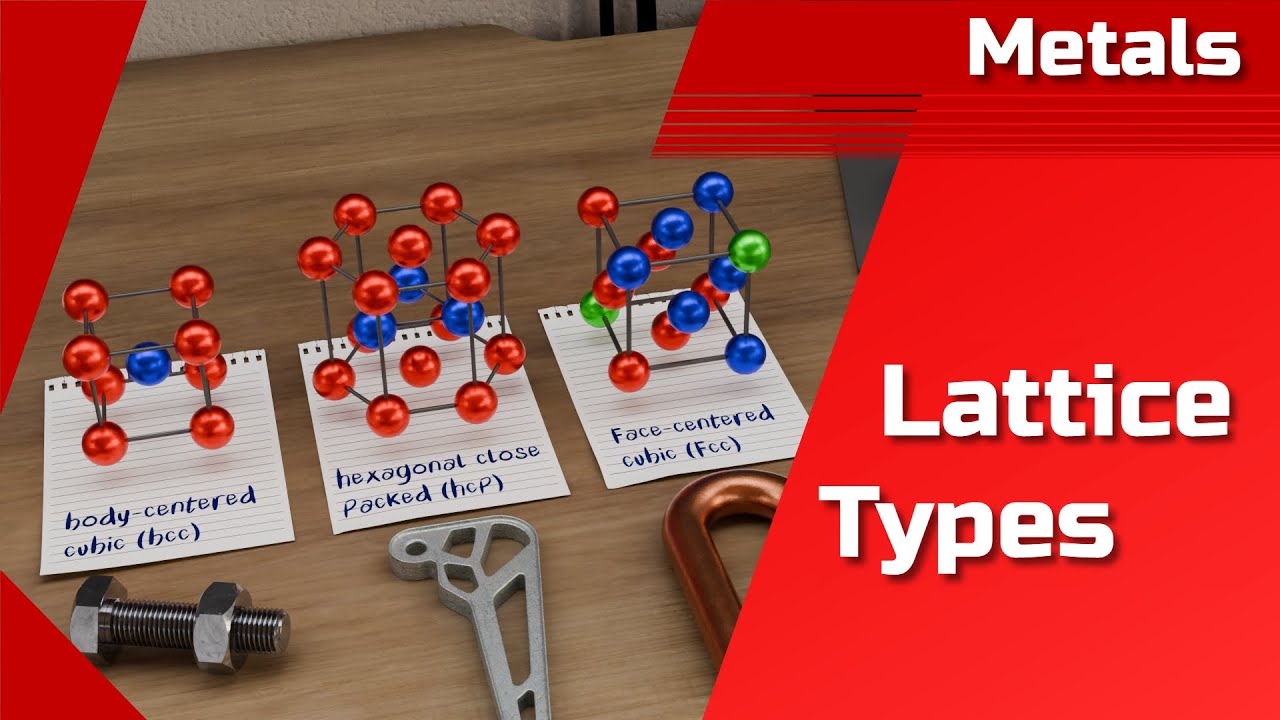
Image taken from the YouTube channel tec-science , from the video titled Structure of metals | lattice types | body-centered cubic, face-centered, hexagonal | bcc, fcc, hcp .
The properties of a material, whether it be the strength of steel in a skyscraper or the conductivity of copper in wiring, are deeply rooted in its atomic arrangement. Understanding these arrangements is paramount in materials science and engineering. At the heart of this understanding lies the concept of crystalline structures.
These structures, characterized by their highly ordered and repeating patterns of atoms, dictate a material’s mechanical, thermal, and electrical behaviors.
Among the various types of crystalline structures, the Body-Centered Cubic (BCC) structure stands out as a fundamental and technologically significant arrangement.
Unveiling the BCC Structure
The BCC structure is a specific type of crystal lattice where atoms are located at each corner of a cube, with an additional atom positioned at the very center of the cube.
This central atom is what distinguishes the BCC structure from simpler cubic lattices. The arrangement leads to unique properties that make BCC metals and alloys indispensable in numerous engineering applications.
Why the BCC Lattice Matters
The BCC lattice isn’t just an abstract concept; it’s a key to understanding the behavior of many essential materials.
Its specific atomic arrangement influences properties like strength, ductility, and melting point, directly impacting how these materials perform in real-world scenarios.
For instance, the BCC structure of iron is crucial to the properties of steel, the backbone of modern construction. Similarly, the high melting point of tungsten, also a BCC metal, makes it ideal for high-temperature applications like light bulb filaments.
Guide Scope and Objectives
This guide aims to provide a comprehensive exploration of the BCC lattice, delving into its fundamental characteristics, its presence in real-world materials, and its influence on material properties.
By understanding the BCC structure, we can better appreciate the link between atomic arrangement and macroscopic material behavior.
From the unit cell configuration to the atomic packing factor, from iron and chromium to strength and ductility, this guide will illuminate the multifaceted nature of the BCC lattice.
The ultimate goal is to equip readers with a solid understanding of the BCC structure and its significance in materials science and engineering.
The BCC lattice isn’t just an abstract concept; it’s a key to understanding the behavior of many essential materials. Its specific atomic arrangement influences properties like strength, ductility, and melting point, directly impacting how these materials perform in real-world scenarios. To truly grasp the significance of the BCC structure, we need to delve into its fundamental characteristics.
BCC Lattice Fundamentals: Unit Cells, Coordination Number, and Atomic Packing Factor
At the heart of understanding any crystal structure lies the concept of its fundamental building block: the unit cell. For the BCC lattice, further crucial characteristics include its coordination number and atomic packing factor.
These properties dictate many of the material’s behaviors, impacting everything from density to deformation mechanisms.
The Body-Centered Cubic Unit Cell
The unit cell is the smallest repeating unit that possesses the full symmetry of the crystal structure. Visualizing and understanding the BCC unit cell is the first step in deciphering its properties.
Visualizing the BCC Unit Cell
Imagine a cube. In the BCC structure, an atom resides at each of the eight corners of this cube. However, what truly defines the BCC structure is the presence of an additional atom located at the very center of the cube.
This central atom doesn’t belong exclusively to this particular unit cell. The corner atoms are shared between eight adjacent unit cells. Only 1/8 of each corner atom effectively belongs to a given unit cell.
Therefore, each BCC unit cell effectively contains two atoms: one from the eight corners (8 corners x 1/8 atom per corner) and one full atom at the body center.
Atom Arrangement in BCC
The atom arrangement in the BCC lattice is characterized by this central atom being equidistant from all eight corner atoms. This arrangement leads to specific interatomic distances and angles, which influence the material’s properties.
The presence of the central atom also significantly affects the packing efficiency and the coordination number, as we will see later.
Coordination Number in the BCC Lattice
The coordination number refers to the number of nearest neighbors that an atom has in a crystal structure. It provides insight into the bonding environment and the strength of atomic interactions.
In the BCC lattice, each atom has a coordination number of eight. The central atom is directly touching all eight corner atoms.
Likewise, each corner atom is touching the central atom of the unit cell and four other corner atoms in the adjacent unit cells.
Influence on Material Properties
The coordination number significantly influences a material’s properties, including its strength and ductility. A higher coordination number generally leads to stronger atomic bonding and increased resistance to deformation.
However, it can also decrease ductility by limiting the ability of atoms to move past each other. The balance between strength and ductility is a crucial consideration in materials design.
Atomic Packing Factor (APF) for BCC
The atomic packing factor (APF) is a measure of how efficiently atoms are packed in a crystal structure. It is defined as the volume of atoms in the unit cell divided by the total volume of the unit cell.
The APF provides insight into the material’s density and the extent of empty space within the structure.
Calculating the APF for BCC
Calculating the APF for BCC involves determining the volume occupied by the two atoms in the unit cell.
Then dividing that value by the total volume of the cubic unit cell.
The APF for BCC is approximately 0.68. This value indicates that about 68% of the space in the BCC structure is occupied by atoms, while the remaining 32% is empty space.
Significance of APF and Comparison with Other Structures
The APF is directly related to the density of the material. A higher APF generally corresponds to a higher density.
Comparing the APF of BCC (0.68) with other common crystal structures like Face-Centered Cubic (FCC) (0.74) and Hexagonal Close-Packed (HCP) (0.74) reveals that BCC is less densely packed.
This lower packing density can influence other properties, such as diffusion rates and the material’s response to stress. The differences in APF between crystal structures contribute to the diverse range of properties observed in different materials.
The implications of the BCC structure extend far beyond theoretical considerations. The arrangement of atoms within this lattice directly translates into tangible properties that make BCC metals and alloys indispensable in a wide array of engineering applications.
Real-World Examples: BCC Metals and Alloys in Engineering Applications
Let’s move beyond the abstract and explore where you’ll encounter BCC structures in the real world, and why they’re chosen for those specific applications. From the structural steel in buildings to the high-performance alloys in jet engines, the BCC lattice plays a critical role.
Common Metals Exhibiting BCC Structure
Several key metals naturally crystallize in the BCC structure, making them fundamental building blocks in engineering. Their inherent properties, dictated by this structure, influence their widespread use.
Iron (α-Fe) and Steel Production
Iron (α-Fe), in its alpha form at room temperature, is perhaps the most economically important BCC metal. Its significance stems primarily from its use as the base constituent in steel production.
The BCC structure of iron provides a framework that can be modified through the addition of carbon and other alloying elements to produce a vast range of steels. These steels possess tailored properties suitable for construction, automotive manufacturing, and countless other applications.
The ability to control the microstructure of steel, largely influenced by the underlying BCC iron lattice, is what makes it such a versatile and essential engineering material.
Chromium and Corrosion Resistance
Chromium is another crucial BCC metal, though it’s rarely used in its pure form. Its primary contribution lies in its exceptional ability to impart corrosion resistance to alloys, particularly stainless steel.
When added to steel, chromium forms a passive layer of chromium oxide on the surface, protecting the underlying metal from oxidation and corrosion.
The BCC structure of chromium contributes to the stability and effectiveness of this protective layer, making it indispensable in environments where corrosion is a major concern, such as chemical processing plants and marine applications.
Tungsten: High Melting Point Applications
Tungsten distinguishes itself with an exceptionally high melting point, the highest of all metals. This characteristic, intrinsically linked to its BCC structure and strong atomic bonding, makes it ideal for high-temperature applications.
Tungsten is a key component in incandescent light bulb filaments, where it withstands extreme temperatures without melting. It’s also used in welding electrodes, high-speed cutting tools, and other applications demanding exceptional thermal stability.
The BCC structure of tungsten ensures that it retains its strength and integrity even at temperatures that would melt other metals.
The Role of BCC Structure in Alloys
While pure BCC metals have their specific uses, their properties can be further enhanced and tailored through alloying. The BCC structure provides a framework for incorporating other elements, leading to a wide range of alloys with optimized characteristics.
BCC Alloys for Specific Properties
Many alloys leverage the BCC structure to achieve specific property combinations. For example, certain titanium alloys adopt a metastable BCC structure at high temperatures, which can be retained at room temperature through rapid cooling.
This allows for subsequent heat treatments to tailor the strength and ductility of the alloy. Similarly, high-entropy alloys, containing multiple principal elements in near-equal proportions, sometimes stabilize in a BCC structure, leading to novel property combinations.
Alloying Effects on Mechanical Properties of BCC Metals
Alloying significantly impacts the mechanical properties of BCC metals. Adding elements like carbon to iron, as in steel, drastically increases its strength and hardness.
This is because the carbon atoms distort the BCC lattice, hindering the movement of dislocations and making it more difficult for the material to deform.
Similarly, alloying can also improve the ductility and toughness of BCC metals by modifying their microstructure and grain size. The specific effects of alloying depend on the elements added and the processing conditions employed.
Chromium is another crucial BCC metal, though it’s rarely used in its pure form. Its primary contribution lies in enhancing the corrosion resistance of steel, forming a passive oxide layer that protects the underlying iron from oxidation.
Its effect on steel’s properties extends beyond corrosion resistance. Chromium significantly influences the steel’s hardness and strength, showcasing the potent effects of even minor alloying additions on a BCC lattice. This leads us to a broader consideration of how the BCC structure itself dictates the mechanical behavior of materials.
Mechanical Properties: Linking BCC Structure to Strength and Ductility
The body-centered cubic (BCC) crystal structure plays a pivotal role in defining the mechanical properties of metals and alloys. Understanding this relationship is critical for materials scientists and engineers seeking to design materials with specific performance characteristics. The arrangement of atoms within the BCC lattice directly influences a material’s strength, ductility, toughness, and overall response to applied stress.
BCC Structure and Strength
BCC metals, in general, exhibit high strength and hardness compared to metals with other crystal structures, such as face-centered cubic (FCC). This inherent strength is attributed to several factors related to the BCC lattice.
The presence of the centrally located atom in the BCC unit cell introduces significant distortion to the lattice structure. This distortion impedes the movement of dislocations, which are line defects that facilitate plastic deformation in crystalline materials.
The more difficult it is for dislocations to move, the stronger and harder the material becomes. The unique slip systems in BCC metals, while numerous, often require higher activation energies, contributing to their strength.
Impact on Ductility and Toughness
While BCC metals are generally strong, they often exhibit lower ductility and toughness compared to FCC metals, particularly at lower temperatures. Ductility refers to a material’s ability to deform plastically before fracture, while toughness describes its resistance to crack propagation.
The limited number of easily activated slip systems in BCC metals can restrict their ability to undergo extensive plastic deformation. This limitation can lead to brittle failure, especially when subjected to impact loading or at low temperatures.
However, it’s crucial to note that the ductility and toughness of BCC metals can be significantly improved through alloying and controlled processing techniques. By carefully selecting alloying elements and optimizing heat treatments, engineers can tailor the microstructure of BCC alloys to enhance their resistance to brittle fracture.
Significance in Materials Science
Understanding the intricate link between the BCC structure and mechanical properties is of paramount importance in materials science and engineering. This knowledge empowers engineers to make informed decisions regarding material selection and design for a wide range of applications.
For instance, the high strength and moderate ductility of BCC steels make them ideal for structural applications where load-bearing capacity is critical.
Conversely, the high-temperature strength and creep resistance of BCC refractory metals, such as tungsten and molybdenum, make them indispensable in aerospace and high-energy applications.
By manipulating the composition and processing of BCC materials, engineers can fine-tune their mechanical properties to meet the specific demands of diverse engineering challenges. The BCC structure, therefore, serves as a foundational element in the design and development of advanced materials.
Frequently Asked Questions About BCC Lattices
Here are some common questions regarding body-centered cubic (BCC) lattices, designed to clarify their structure and properties.
What exactly defines a BCC lattice meaning?
The BCC lattice meaning refers to a specific type of crystal structure where atoms are arranged in a cubic pattern with one atom at each corner of the cube and one atom positioned at the center of the cube’s body. This central atom significantly influences the material’s properties.
How does the BCC lattice meaning affect material properties?
The presence of the central atom in the BCC lattice meaning directly impacts its strength and ductility. It makes the material generally stronger than those with a simple cubic structure but less ductile than those with a face-centered cubic (FCC) structure.
Can you give some examples of metals with a BCC lattice?
Several common metals crystallize in a BCC lattice structure. Examples include iron (at room temperature), chromium, tungsten, and vanadium. Their BCC lattice meaning plays a crucial role in their widespread industrial applications.
How does the atomic packing factor relate to the BCC lattice meaning?
The atomic packing factor (APF) is a measure of how efficiently atoms fill the space in a crystal structure. For a BCC lattice, the APF is approximately 0.68. This value indicates that the BCC lattice meaning dictates that about 68% of the space is occupied by atoms.
Hopefully, this deep dive into bcc lattice meaning cleared things up for you! Now go forth and impress everyone with your newfound knowledge of crystal structures.